Hydrogen gas and oxygen gas react to form water, and this reaction can be depicted as follows:
Question:
Hydrogen gas and oxygen gas react to form water, and this reaction can be depicted as follows:
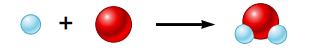
Explain why this equation is not balanced, and draw a picture of the balanced equation.
Transcribed Image Text:
+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
It is because hydrogen has a much larger mass than ox...View the full answer

Answered By

Willis Omondi
Hi, I'm Willis Omondi, a proficient and professional academic writer. I have been providing high-quality content that best suits my clients and completing their work within the deadline. All my work has been 100% plagiarism-free, according to research from my services, especially in arts subjects and many others
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Draw a picture of the following cell and write reduction half- reactions for each electrode: Pt(s) | Fe*(aq), Fe*(aq) || Cr,O3 (aq), Cr*(aq), HA(aq) | Pt(s)
-
A photographer takes a picture of the Washington Monument. The next day, another photographer takes an identical picture of the Washington Monument. Which picture is entitled to copyright protection?...
-
Methane (CH4) and oxygen (O2) react to form formaldehyde (CH2O) (reaction #1), with a competing side reaction (#2) in which methane and oxygen form carbon dioxide (CO2): CH4(v) + O2(g) -> CH2O(v)...
-
The manufactured wood beam carries a uniformly distributed load of in- tensity wo. Determine the largest safe value of wo if the maximum shear stress in the wood is limited to 300 psi. 1.0 ft Wo 4 ft...
-
Explain what acquisition indebtedness and home equity indebtedness are with respect to a qualified residence of a taxpayer. Identify any limitations on the deductibility of interest expense on this...
-
The wall hanger has a thickness of 0.25 in. and is used to support the vertical reactions of the beam that is loaded as shown. If the load is transferred uniformly to each strap of the hanger,...
-
Red meat and heart disease. Refer to the study presented at ESC Preventive Cardiology 2021 that explored the link between red meat and heart disease, Exercise 9.12 (p. 533). Recall that the study...
-
(a) What specific recommendations would you give the Johnsons for selecting checking and savings accounts that will enable them to effectively use the first and second tools of monetary asset...
-
Please help! On January 1, 2017, a subsidiary sold equipment to its parent for $520,000. The subsidiary's original cost was $200,000 and as of January 1, 2017, $20,000 in depreciation had been...
-
The table below shows Crystal's total cost of producing different quantities of tie-dyed t-shirts for a local arts festival. Instructions: Enter your answers as a whole number. a. Complete the...
-
Which (if any) of the following is(are) true regarding the limiting reactant in a chemical reaction? a. The limiting reactant has the lowest coefficient in a balanced equation. b. The limiting...
-
In chemistry, what is meant by the term mole? What is the importance of the mole concept?
-
Determine the integrals in Exercises by making appropriate substitutions. f= V X dx
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{D}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
Draw a line graph for each data set in Problems 36-39. Data set A Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 25 16 25 25 14 18 1 2 2 2...
-
For each of the angles shown: (i) Estimate its size (ii) Measure it and check how good your estimate was. Aim for your estimate to be within 10 of the actual angle. a. b. c. d. e. f.
-
For the quasispin model of Problem 31.1 , find the eigenvalues of $s_{0}^{(m)}$ for the levels labeled by $m$. Show that the system has a total quasispin $S$ that is the vector sum of quasispins for...
-
A sole proprietorship was started on January 1, 2005, when it received \($60,000\) cash from Mark Pruitt, the owner. During 2005, the company earned \($40,000\) in cash revenues and paid \($19,300\)...
-
Assume that the economy is at potential output and the natural rate of unemployment and that inflation is at the target rate set for the central bank by the government. In each of the following...
-
Solve for the equilibria of the following discrete-time dynamical systems Pr pt+1 = Pr+2.0(I-Pr)
-
A gas mixture consists of 320 mg of methane, 175 mg of argon, and 225 mg of neon. The partial pressure of neon at 300 K is 8.87 kPa. Calculate (a) The volume and (b) The total pressure of the mixture.
-
In an experiment to measure the molar mass of a gas, 250 cm3 of the gas was confined in a glass vessel. The pressure was 152 Torr at 298 K and, after correcting for buoyancy effects, the mass of the...
-
A certain sample of a gas has a volume of20.00 dm ' at OCand 1.000 atm. A plot of the experimental data of its volume against the Celsius temperature, , at constant p, gives a straight line of slope...
-
The major justification for adding Step 0 to the U.S. GAAP impairment test for goodwill and indefinite lived intangibles is that it: A. Saves money spent estimating fair values B. Results in more...
-
Regarding research and experimental expenditures, which of the following are not qualified expenditures? 3 a. costs of ordinary testing of materials b. costs to develop a plant process c. costs of...
-
Port Ormond Carpet Company manufactures carpets. Fiber is placed in process in the Spinning Department, where it is spun into yarn. The output of the Spinning Department is transferred to the Tufting...

Study smarter with the SolutionInn App


