Match each name below with the following microscopic pictures of that compound in aqueous solution. a. Barium
Question:
Match each name below with the following microscopic pictures of that compound in aqueous solution.
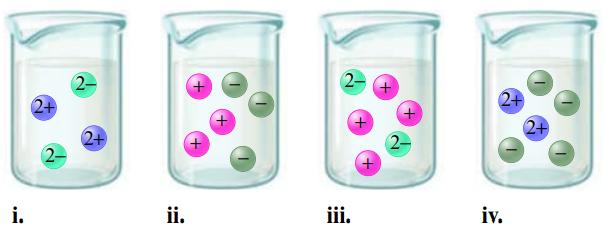
a. Barium nitrate
b. Sodium chloride
c. Potassium carbonate
d. Magnesium sulfate
Which picture best represents HNO3(aq)? Why aren’t any of the pictures a good representation of HC2H3O2(aq)?
Transcribed Image Text:
i. 2+ 2- 2- 2+ ii. 2- + 2- 2+ iv.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
ANSWER i d since both MgMg2 and sulfate SO42 are divalent ions iib since both NaNa and ClCl ar...View the full answer

Answered By

Dennis Nyangau
I have been tutoring for several years now, and I absolutely love it! I love being able to help students one-on-one and see them succeed. It is so gratifying to see a student understand a concept that they were struggling with before. I also enjoy getting to know my students and helping them to reach their full potential.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Match each description below with the following microscopic pictures. More than one picture may fit each description. A picture may be used more than once or not used at all. a. A gaseous compound b....
-
Match the crystal field diagrams given below with the following complex ions. a. b. c. ( assume strong ie ld) (assume weak field)
-
Match the crystal field diagrams given below with the following complex ions. a. b. [Fe(CN)6]3- [Mn(H2O)6l2+
-
Look at the expansion joint in the photo of Figure 15.13. Would you say the photo was taken on a warm day or a cold day? Why?
-
Retailers suffer extensive losses due to inventory shrinkage or shoplifting. This is a very real cost that is passed on to all virtuous shoppers who have no intention of engaging in theft. The tort...
-
Consider the NFL data in Exercise 12-21. (a) Test for significance of regression using α = 0.05. What is the P-value for this test? (b) Conduct the t-test for each regression...
-
Have you noticed how your age or gender affects the way you communicate or the way others communicate with you? Describe and explain.(pp. 102103)
-
The manager of a minor league baseball team wants to estimate the average fastball speed of two pitchers. He clocks 50 fastballs, in miles per hour, for each pitcher. A portion of the data is shown...
-
Chapter 7: Inventory Additionally, please refer to Chapter 7 in your Cengage Accounting eText, accessible from the eText link in the Course Navigation Panel to the left of your screen. Requirement 1:...
-
Twenty-five years ago, Angelo and Fred started their own consulting company, XYZ Co. Angelo, who is 55, retired from the business on December 31, 2019. He and his wife plan to travel throughout...
-
Arrange the following substances in order of increasing mass percent of carbon. a. Caffeine, C 8 H 10 N 4 O 2 b. Sucrose, C 12 H 22 O 11 c. Ethanol, C 2 H 5 OH
-
What number of atoms of nitrogen are present in 5.00 g of each of the following? a. glycine, C 2 H 5 O 2 N b. Magnesium nitride c. Calcium nitrate d. Dinitrogen tetroxide
-
What financial statements must be presented by a government university that engages in both governmental and business-type activities? Discuss.
-
Based on the case, Insights Analytics: Technology for a Knowledge Management Program attached . Please explain all 8 points. Explanation of each point should be 300words . Please attach the reference...
-
When women were finally allowed to become pilots of fighter jets, engineers needed to redesign the ejection seats because they had been originally designed for men only. The ejection seats were...
-
What will be the output of the following code snippet? with open ("hello.txt", "w") as f: f.write("Hello World how are you today") with open('hello.txt', 'r') as f: data = f.readlines () for line in...
-
Assume that females have pulse rates that are normally distributed with a mean of p = 72.0 beats per minute and a standard deviation of o = 12.5 beats per minute. Complete parts (a) through (c)...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
Solve the system of equations using matrices (row operations). If the system has no solution, say that it is inconsistent. y = 7 9 %3D 21
-
[a] Two foam blocks, each with a charge of 19 micro coulombs (1 C = 10-6 C), are both held in place 19 cm apart in the east-west direction. A foam ball with a charge 49 C is placed 55 cm north of the...
-
A student intends to titrate a solution of a weak monoprotic acid with a sodium hydroxide solution but reverses the two solutions and places the weak acid solution in the buret. After 23.75 mL of the...
-
A 0.210-g sample of an acid (molar mass = 192 g/mol) is titrated with 30.5 mL of 0.108 M NaOH to a phenolphthalein end point. Is the acid monoprotic, diprotic, or triprotic?
-
A certain acetic acid solution has pH = 2.68. Calculate the volume of 0.0975 M KOH required to reach the equivalence point in the titration of 25.0 mL of the acetic acid solution.
-
The plant asset and accumulated depreciation accounts of Pell Corporation had the following balances at December 3 1 , 2 0 2 0 : Transactions during 2 0 2 1 were as follows: a . On January 2 , 2 0 2...
-
All else equal, a company's P/E ratio will ____________ when the discount rate ___________. rise, rise fall, rise fall, falls cannot be determined All else equal, a company's P/E ratio will _____...
-
The cost of partially completed goods at the end of the period would be Ending work in process inventory Cost of goods sold Beginning finished goods inventory Beginning work in process inventory

Study smarter with the SolutionInn App


