Name the compounds in parts ad and write the formulas for the compounds in parts eh. a.
Question:
Name the compounds in parts a–d and write the formulas for the compounds in parts e–h.
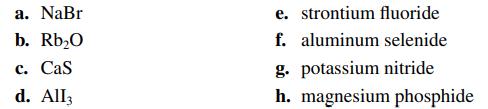
Transcribed Image Text:
a. NaBr b. Rb₂0 c. Cas d. All3 e. strontium fluoride f. aluminum selenide g. potassium nitride h. magnesium phosphide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
a NaBr Sodium bromide b RbO Rubidium oxi...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Write formulas for and name the binary hydrogen compounds of the second-period elements (Li to F). Describe how the physical and chemical properties of these compounds change from left to right...
-
Write expanded formulas for the following compounds and name them using the IUPAC system: a. (CH3)3CCH2CH2CH3 b. CH3(CH2)2CH3 c. (CH3)2CHCH2CH2CH3 d. CH3CCl2CF3 e. (CH2)4 f. CH3CH2CHFCH3 g. EtBr h....
-
Write the molecular and structural formulas for the compounds represented by the following molecular models: (a) (b) (c) (d) P F F F
-
1. If net profit is $47,025; depreciation is $2,200; accounts receivable increases $5,056; accounts payable increases $4,673; and inventory declines $850; what is the operating cash flow for the...
-
Pamello, Inc., an engineering consulting firm, uses the cash method of accounting and is a calendar year taxpayer. Compute the amount of Pamellos current year deductions for the following...
-
Does it make sense to you that a leader should develop an individualized relationship with each follower? Explain advantages and disadvantages to this approach.
-
Job performance under time pressure. Refer to the Academy of Management Journal (October 2015) study of how time pressure affects team job performance, Exercise 12.89 (p. 768). Recall that the...
-
The adjusted trial balance for Anara Co. as of December 31, 2013, follows. P. Anara invested $40,000 cash in the business during year 2013 (the December 31, 2012, credit balance of the P. Anara,...
-
Onandomba Pty (Ltd) provides skilled labour to the construction of low-cost apartments. They have recently been asked by a builder NHE to bid for a kitchen fitting contract for a new development of...
-
A URL is a type of _________. a. Web page b. URI c. Link d. Network
-
Complete the following table. Atom/lon 50 Sn 25Mg+ 2+ 56 Fe+ 120S 79 32 Se 34- 35C1 53 Cu Protons Neutrons Electrons
-
For carbon-14 and carbon-12, how many protons and neutrons are in each nucleus? Assuming neutral atoms, how many electrons are present in an atom of carbon-14 and in an atom of carbon-12?
-
Would it be appropriate to define the policy of quantitative easing as monetarist?
-
The amounts of caffeine in a sample of five-ounce servings of brewed coffee are shown in the histogram. Number of 5-ounce servings S 25- 20 15 10 25 12 10 1 2 70.5 92.5 114.5 136.5 158.5 Caffeine (in...
-
Tom, David, Dale, and Murdock are four business students who want to rent a four- bedroom apartment together for the fall semester. They have identified the three factors important to them in...
-
Listed below, out of order, are the steps in an accounting cycle. 1. Prepare the unadjusted trial balance. 2. Post journal entries to general ledger accounts. 3. Analyze transactions from source...
-
Consider Quick Start QFD Matrix 2 above. Which two technical specifications are strongly correlated with each other? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation ==...
-
A cylindrical solenoid of length \(\ell\) and radius \(R\) has \(n\) windings per unit length and carries a current \(I\). (a) Use the inductance expression \(L=\left(\mu_{0} N^{2} A ight) / \ell\)...
-
1. How is the existence of surveys of business confidence likely to affect firms expectations and actions? 2. Why, if the growth in output slows down (but is still positive), is investment likely to...
-
Chloroplasts are illuminated until the levels of the Calvin cycle intermediates reach a steady state. The light is then turned off. How does the level of RuBP vary after this point?
-
The 1980s saw reports of fH (SiH2) ranging from 243 to 289 k] mol-1. For example, the lower value was cited in the review article by R. Walsh (Ace. Chem. Res. 14,246 (1981)); Walsh later leant...
-
Given that tG = -212.7 kJ mol-1 for the reaction in the Daniell cell at 25C, and b (CuS04) = 1.0 x 10-3 mol kg-i and b (ZnS04) = 3.0 x 10-3 mol kg-I, calculate (a) The ionic strengths of the...
-
Although the hydrogen electrode may be conceptually the simplest electrode and is the basis for our reference state of electrical potential in electrochemical systems, it is cumbersome to use....
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


