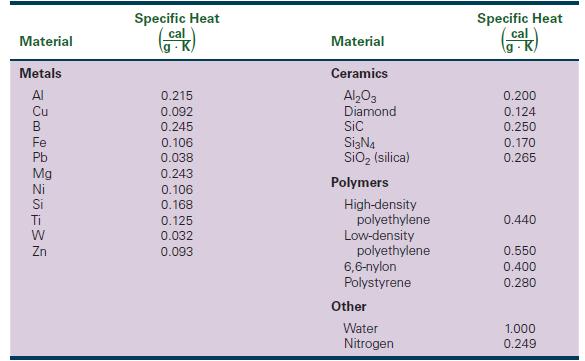
Of the metals listed in Table 22-1, which have the highest and lowest specific heats? Also list
Question:
Of the metals listed in Table 22-1, which have the highest and lowest specific heats? Also list their atomic masses
Transcribed Image Text:
Material Metals Al Cu B Fe Pb Mg Ni Si Ti W Zn Specific Heat cal 0.215 0.092 0.245 0.106 0.038 0.243 0.106 0.168 0.125 0.032 0.093 Material Ceramics Al₂O3 Diamond Sic Si3N4 SiO₂ (silica) Polymers High-density polyethylene Low-density polyethylene 6,6-nylon Polystyrene Other Water Nitrogen Specific Heat cal K 0.200 0.124 0.250 0.170 0.265 0.440 0.550 0.400 0.280 1.000 0.249
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
ANSWER From the table we can see that the metal with the highest specific heat is magnesium Mg ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Of the metals listed in Table 19-1, which are the most and least conductive? Material Superconductors Hg, Nb3Sn YBaCu3O7-x MgB Metals Alkali metals Na K Alkali earth metals Mg Ca Group 3B metals Al...
-
In Figure 20-9(b), what materials have the highest and lowest saturation magnetizations and coercivities? Square loop for computer applications Inductance (a) Soft magnet for electrical applications...
-
For each of the metals listed in the following table, compute the Pilling-Bedworth ratio. Also, on the basis of this value, specify whether you would expect the oxide scale that forms on the surface...
-
Which of the following are valid in a Java file, listed in the order in which they are declared? (Choose two.) A. A package-private class declaration and a public interface declaration B. Two package...
-
In the business world of the 21st Century, will it be possible to make critical marketing decisions without marketing research? Why or why not?
-
A highly viscous liquid discharges from a large container through a small-diameter tube in laminar flow. Disregarding entrance effects and velocity heads, obtain a relation for the variation of fluid...
-
Who are the principal channel members in lodging distribution?
-
Sulfur dioxide is oxidized to sulfur trioxide in a small pilot-plant reactor. SO 2 and 100% excess air are fed to the reactor at 450C. The reaction proceeds to a 65% SO 2 conversion, and the products...
-
Superior Company provided the following data for the year ended December 31 (all raw materials are used in production as direct materials): Selling expenses $ 214,000 Purchases of raw materials $...
-
Thinking back to high school or first year chemistry, you may recall how to calculate mole fractions. For example, if you have 1 kmol of two liquid species (A and B), in total you have 2 kmol, and...
-
Calculating Power in Decibels. In an optical communications system or electrical power transmission system, the power or signal often is transferred between several components. The decibel (dB) is a...
-
What is the minimum accelerating voltage required to produce Ka x-rays in nickel?
-
Among external stakeholders, whats the difference between the task environment and the general environment?
-
Gil and Ruth George have been friends of yours for many years. They have come to you for advice on their estate plan since they want a second opinion to make sure it is going to do what they hope....
-
The test statistic of z = 1.74 is obtained when testing the claim that p # 0.658. Identify the hypothesis test as being two-tailed, left-tailed, or right-tailed. Find the P-value. Using a...
-
Ann and Bob had their first date. Each either felt romantic chemistry (C) or no chemistry (NC) with the other person. Each person knows his/her own feeling but does not know the feeling of the other...
-
Find the following using countif, countifs, sumif, sumifs, averageif, and averageifs. Create all formulas and calculations directing in Excel. How many songs are sung by Moore? What is the average...
-
Total number of Ledgers, Groups, Entries etc. can be shown from o a. Tally Audit o b. Statistics o c. Accounts Information o d. Company Information
-
Find the remaining angle(s) and side(s) of triangle, if it (they) exists. If no triangle exists, say No triangle. a = 2, b = 3, A = 20
-
According to a recent survey, 40% of millennials (those born in the 1980s or 1990s) view themselves more as spenders than savers. The survey also reveals that 75% of millennials view social...
-
As quality control engineer for your company, you must approve all material shipments from your suppliers. Part of this job involves testing random samples from each delivery and making sure they...
-
When estimating the live load for a new bridge design, you want that estimate to be conservative. In other words, you want to error on the safe side by basing the estimate on the worst possible...
-
Determine the forces acting on the ends of the rope by (1) drawing the free-body diagram, (2) counting unknowns and equations to check determinacy, (3) writing the equilibrium equations, and (4)...
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...
-
What is the Breakeven Point in units assuming a product selling price is $100, Fixed Costs are $8,000, Variable Costs are $20, and Operating Income is $32,000 ? 100 units 300 units 400 units 500 units

Study smarter with the SolutionInn App


