One method for determining the purity of aspirin (C 9 H 8 O 4 ) is to
Question:
One method for determining the purity of aspirin (C9H8O4) is to hydrolyze it with NaOH solution and then to titrate the remaining NaOH. The reaction of aspirin with NaOH is as follows:
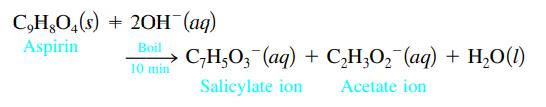
A sample of aspirin with a mass of 1.427 g was boiled in 50.00 mL of 0.500 M NaOH. After the solution was cooled, it took 31.92 mL of 0.289 M HCl to titrate the excess NaOH. Calculate the purity of the aspirin. What indicator should be used for this titration? Why?
Transcribed Image Text:
C,H,O4(s) + 2OH(aq) Aspirin Boil C₂H5O3(aq) + C₂H₂O₂ (aq) + H₂O(1) 10 min Salicylate ion Acetate ion
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Solution a The purity of the aspirin NaOH x ml NaOH used to titrate excess mL HCl used 0500 M x ...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What is the equation method for determining the break-even point? Explain how the results of this method differ from those of the contribution margin approach.
-
Determining Diet One method for determining the amount of com in early Native American diets is the stable isotope ratio analysis (SIRA) technique. As com photosynthesizes, it concentrates the...
-
One method for determining the position of an image, either real or virtual, is by means of parallax. If a finger or other object is placed at the position of the image, as shown in Figure Q36.25,...
-
The Best Buy Co. Inc. 10-K report has the following footnote related to leasing activities. The future minimum lease payments under our capital and operating leases by fiscal year (not includ- ing...
-
Has the availability of shelf registrations reduced the importance of private placements? Why?
-
Figure 24.15 shows the client and server in the transition diagram for the common scenario using a four-handshake closing. Change the diagram to show the three-handshake closing.
-
To assess the current legal risks associated with serving alcohol. AppendixLO1
-
What proportion of secretaries of Fortune 500 companies has a personal computer at his or her workstation? You want to answer this question by conducting a random survey. How large a sample should...
-
A put option that expires in six months with an exercise price of $65 sells for $5.00. The stock is currently priced at $61, and the risk-free rate is 3.7 percent per year, compounded continuously....
-
A small engineering firm has 4 senior designers available to work on the firms 4 current projects over the next 2 weeks. The firms manager has developed the following table of quality scores, which...
-
What quantity (moles) of HCl(g) must be added to 1.0 L of 2.0 M NaOH to achieve a pH of 0.00? (Neglect any volume changes.)
-
You make 1.00 L of a buffered solution (pH = 4.00) by mixing acetic acid and sodium acetate. You have 1.00 M solutions of each component of the buffered solution. What volume of each solution do you...
-
Two men stand 10 m apart on level ground near the edge of a cliff. One man drops a stone and one second later the other man drops a stone. One second after that, how fast is the distance between the...
-
Use the information below to answer the next question. Below are different graphs that could represent the magnitude of an Electric Field from a source. Teza E Distance E 4 Tza E Taza 2 Distance 5 3...
-
Factor out the GCF: 36c5 +54c8
-
Demonstrate that a circle with a radius of r has a circumference of 2 pi ( r ) . HINT: Begin by examining the equation for the upper semicircle, utilize the arc length formula, and then double the...
-
Graph the function f(x) = 3.x - 7.
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
Show that the difference quotient for f(x) = sin x is given by f(x + h) - f(x) sin(x + h) sin x 1- cos h sin x sin h = Cos X
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
When acetic acid is treated with isotopically labeled water ( 18 O, shown in red) in the presence of a catalytic amount of acid, it is observed that the isotopic label becomes incorporated at both...
-
Phosgene is highly toxic and was used as a chemical weapon in World War I. It is also a synthetic precursor used in the production of many plastics. (a) When vapors of phosgene are inhaled, the...
-
Fluphenazine is an antipsychotic drug that is administered as an ester prodrug via intramuscular injection: The hydrophobic tail of the ester is deliberately designed to enable a slow release of the...
-
explain in excel please For a particular product the price per unit is $6. Calculate Revenue if sales in current period is 200 units. Conduct a data analysis, on revenue by changing the number of...
-
Hall Company sells merchandise with a one-year warranty. In the current year, sales consist of 35,000 units. It is estimated that warranty repairs will average $10 per unit sold and 30% of the...
-
Q 4- Crane Corporation, an amusement park, is considering a capital investment in a new exhibit. The exhibit would cost $ 167,270 and have an estimated useful life of 7 years. It can be sold for $...

Study smarter with the SolutionInn App


