Sketch the galvanic cells based on the following overall reactions. Show the direction of electron flow, the
Question:
Sketch the galvanic cells based on the following overall reactions. Show the direction of electron flow, the direction of ion migration through the salt bridge, and identify the cathode and anode. Give the overall balanced equation. Assume that all concentrations are 1.0 M and that all partial pressures are 1.0 atm.
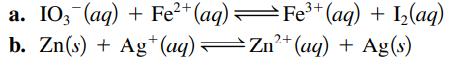
Transcribed Image Text:
2+ a. IO3(aq) + Fe²+ (aq) — Fe³+ (aq) + 1₂(aq) = b. Zn(s) + Ag+ (aq) Zn²+ (aq) + Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
ANSWER a The galvanic cell for the specified overall re...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Sketch the galvanic cells based on the following half-reactions. Calculate Ïo, show the direction of electron flow and the direction of ion migration through the salt bridge, identify the...
-
Sketch the galvanic cells based on the following overall reactions. Calculate o , show the direction of electron flow and the direction of ion migration through the salt bridge, identify the cathode...
-
Assume both electron and hole concentrations in a semiconductor are raised by n above their equilibrium values. Define a net minority carrier lifetime t by R = n/t. give expressions for t in terms of...
-
Which of the following statements about an acquiescence is correct? a. Acquiescences are published only for certain regular decisions of the U.S. Tax Court. b. The IRS does not issue acquiescences to...
-
Robert Shiller of Yale University has suggested a variation on ARMs in which mort-gage interest rates are tied to inflation, not to short-term interest rates. Discuss the pros and cons of this idea...
-
The transfer function of a filter is (a) Find the poles and zeros of H(s) and use this information to sketch the magnitude response |H(jΩ)|of the filter. Indicate the magnitude response...
-
If consideration paid to acquire an interest in a partnership is based on the fair value of the net assets, why doesnt the bonus method recognize all of the suggested values? AppendixLO1
-
Valmont, Inc., experienced the following events in 2012, in its first year of operation. 1. Received $20,000 cash from the issue of common stock. 2. Performed services on account for $50,000. 3. Paid...
-
Please help me solve! During 2020, Kaleb, a 40-year-old single taxpayer, reports the following items of income and expense: (Click the icon to view the data.) (Click the icon to view the standard...
-
Write the balanced equation for the reaction of iron(II) ion with nitrate ion in acidic solution. Nitrate ion is reduced to NO.
-
Calculate G and K at 25 C for the reactions in Exercises 37 and 41. Data in Exercise 37 Sketch the galvanic cells based on the following overall reactions. Show the direction of electron flow, and...
-
Which of the following statements concerning corrosion is(are) true? For the false statements, correct them. a. Corrosion is an example of an electrolytic process. b. Corrosion of steel involves the...
-
Powers Wrecking Service demolishes old buildings and other structures and sells the salvaged materials. During 2019, Powers had $425,000 of revenue from demolition services and $137,000 of revenue...
-
Notation Using the weights (Ib) and highway fuel consumption amounts (mi/gal) of the 48 cars listed in Data Set 35 "Car Data" of Appendix B, we get this regression equation: = 58.9 - 0.00749x, where...
-
Week 11-Final Exam: Chapters 5-7 Question 15 of 30 -135 Current At in Ppm 06-20 10%.onthe 1110077 OORE Textbook and M DOLL F T 19 19 Q w A R T Y 3 . 9 4 S D 4 G H A L x N M Cu T
-
We have two samples: sample 1 n= 39 -X= 98.2 S= 15.9 sample 2 n=31 -X=119.2 S= 23.0 begin testing whether u1
-
Discuss charitable purpose trusts under Section 3(1), Charities Act 2011.
-
Amadeus Corporation is considering the issue of a new product to be added to its product mix. They hired you, a recent business graduate from MacEwan, for conducting the analysis. The production line...
-
Solve the equation. Give solutions in exact form. 5 ln x = 10
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
If an electron passes through an electrical potential difference of 1 V, it has an energy of 1 electron-volt. What potential difference must it pass through in order to have a wavelength of 0.300 nm?
-
Calculate the longest and the shortest wavelength observed in the Balmer series.
-
X-rays can be generated by accelerating electrons in a vacuum and letting them impact on atoms in a metal surface. If the 1250. eV kinetic energy of the electrons is completely converted to the...
-
The following amounts were reported on the December 31, 2022, balance sheet: Cash $ 8,000 Land 20,000 Accounts payable 15,000 Bonds payable 120,000 Merchandise inventory 30,000 Retained earnings...
-
Sandhill Co. issued $ 600,000, 10-year, 8% bonds at 105. 1.Prepare the journal entry to record the sale of these bonds on January 1, 2017. (Credit account titles are automatically indented when the...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.

Study smarter with the SolutionInn App


