Specify which of the following are oxidationreduction reactions, and identify the oxidizing agent, the reducing agent, the
Question:
Specify which of the following are oxidation–reduction reactions, and identify the oxidizing agent, the reducing agent, the substance being oxidized, and the substance being reduced.
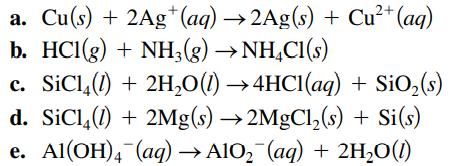
Transcribed Image Text:
2+ a. Cu(s) + 2Ag+ (aq) →2Ag(s) + Cu²+ (aq) b. HCl(g) + NH3(g) →NH4Cl(s) c. SiC¹4(1) + 2H₂O(l) →4HCl(aq) + SiO₂ (s) d. SiC14 (1) + 2Mg(s) →2MgCl₂(s) + Si(s) e. Al(OH)4 (aq) →AlO₂ (aq) + 2H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a This is an oxidationreduction reaction Oxidizing ...View the full answer

Answered By

Hande Dereli
Enthusiastic tutor, skilled in ACT and SAT tutoring. Raised one student's score on the SATs from 1100 combined to 1400. Graduated with a 3.9 GPA from Davidson College and led a popular peer tutoring group for three years. Scored in the top 0.06% in the nation on the SATs. The real reason I'm the one to help you nail the test? Results. Clients invariably praise my ability to listen and communicate in a low-stress, fun way. I think it's that great interaction that lets me raise retest SAT scores an average of 300 points.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Specify which of the following elements you would expect to have the greatest electron affinity and which would have the least: He, K, Co, S, Cl.
-
Identify with a which of the following are subdivisions of owners equity. a. Vehicles _____ b. J. Penny, Capital _____ c. Accounts Payable _____ d. J. Penny, Withdrawals _____ e. Accounts Receivable...
-
Identify with a which of the following are subdivisions of owners equity. a. Land b. M. Kaminsky, Capital c. Accounts Receivable d. M. Kaminsky, Withdrawals _____ e. Accounts Payable _____ f. Rent...
-
What conditions must be met for an award to qualify for an exclusion under Sec. 74?
-
Arrange the following data storage concepts in order from smallest to largest, in terms of their size: file, record, database, character, and field.
-
A company manufactures a single product. The standard mix is as under: Material A 60% at Rs. 20 per kg Material B 40% at Rs. 10 per kg Normal loss in production is 20% of input. Due to shortage of...
-
Does the airlines current strategy truly differentiate it from its competitors? Is the strategy sustainable? Its the same plane going to the same place at exactly the same time. But these days, not...
-
Problem 7-4A a-c (Video) At the beginning of last year (2019), Richter Condos installed a mechanized elevator for its tenants. The owner of the company, Ron Richter, recently returned from an...
-
A manufacturing firm produces diesel engines in four citiesPhoenix, Seattle, St. Louis, and Detroit. The company is able to produce the following numbers of engines per month: Plant .. Production 1....
-
A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M HNO 3 solution. If the unknown base concentration is 0.0300 M, give some possible identities for...
-
A 25.00-mL sample of hydrochloric acid solution requires 24.16 mL of 0.106 M sodium hydroxide for complete neutralization. What is the concentration of the original hydrochloric acid solution?
-
Prove that the inverse of a (nonsingular) symmetric matrix is symmetric.
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
X 10 Let A = -9 y 7 4 Z 210 If the kernel of A contains the vector what are x, y, and z? -2
-
8-22. E.O.Q., Carrying cost = Storing cost + Interest. Following data are available with respect to a certain material. Annual requirement.......... Cost to place an order.. Annual interest rate. _...
-
A new company started production. Job 1 was completed, and Job 2 remains in production. Here is the information from the job cost sheets from their first and only jobs so far: Job 1 Hours Total Cost...
-
What factors could account for the decline in union membership in recent years?
-
What key concerns must functional tactics address in marketing? Finance? POM? Personnel?
-
The centre of the EPR spectrum of atomic deuterium lies at 330.02 mT in a spectrometer operating at 9.2482 GHz. What is the g-value of the electron in the atom?
-
A radical containing three equivalent protons shows a four-line spectrum with an intensity distribution 1:3:3:1. The lines occur at 331.4 mT, 333.6 mT, 335.8 mT, and 338.0 mT. What is the hyperfine...
-
A radical containing three in equivalent protons with hyperfine constants 2.11 mT, 2.87 m'T and 2.89 mT gives a spectrum centred on 332.8 mT. At what fields do the hyperfine lines occur and what are...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...
-
Fibertech GmbH is a distributor of outdoors technical clothing. The company outsources the production of clothing to external manufacturers in Bangladesh and sells the clothing under its own brands....

Study smarter with the SolutionInn App


