Sulfur trioxide, SO 3 , is produced in enormous quantities each year for use in the synthesis
Question:
Sulfur trioxide, SO3, is produced in enormous quantities each year for use in the synthesis of sulfuric acid.
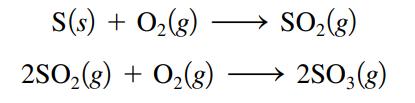
What volume of O2(g) at 350.οC and a pressure of 5.25 atm is needed to completely convert 5.00 g sulfur to sulfur trioxide?
Transcribed Image Text:
S(s) + 0₂ (8) + O₂(g) 2SO₂(g) SO₂(g) 2SO3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The volume 500 g x 1 mol SO38409 g SO3 20 moles O21 mol O2 0...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
An ideal gas at 15.5 C and a pressure of 1.72 105 Pa occupies a volume of 2.81 m3. (a) How many moles of gas are present? (b) If the volume is raised to 4.16 m3 and the temperature raised to 28.2...
-
Air at a temperature of 30 C and a pressure of 100 kPa has a relative humidity of 80%. Calculate (a) The molal humidity of the air (b) Saturated molal humidity of this air if its temperature is...
-
5 liters of air with a humidity of 0.6, a temperature of 20 C and a pressure of 75 cm Hg it is pressed together to 2 liters at a temperature of 25 C. Calculate all missing state parameters, the...
-
Write a function my_ieee_2_dec(ieee), where icce is a string contains 64 char- acters of ones and zeros representing a 64-bit IEEE754 number. The output should be d, the equivalent decimal...
-
Adriana Santosh is in the business of selling antique furniture. As her company has increased significantly the Internet portion of their business, Adriana has determined that she does not need as...
-
Three different pesticides can be used to control pest infestation of grapes. It is suspected that pesticide 3 is more effective than the other two. In a particular vineyard, three different...
-
9 What is the difference between a cognitive and a communal view of knowledge?
-
Mr. Lion, who is in the 37 percent tax bracket, is the sole shareholder of Toto, Inc., which manufactures greeting cards. Totos average annual net profit (before deduction of Mr. Lions salary) is...
-
make sure to answer all the questions in the tab Required information The following information applies to the questions displayed below.) One Product Corporation (OPC) incorporated at the beginning...
-
Can we conclude that the mean age at death of patients with homozygous sickle-cell disease is less than 30 years? A sample of 50 patients yielded the following ages in years: Let alpha = 0.05. 1.7...
-
A typical adult inhales 450 mL of air in any one breath. How many air particles are in a typical breath at 745 torr and 22C?
-
A 2.50-L container is filled with 175 g argon. a. If the pressure is 10.0 atm, what is the temperature? b. If the temperature is 225 K, what is the pressure?
-
The following questions are used in the Kaplan CPA Review Course to study accounting for income taxes while preparing for the CPA examination. Determine the response that best completes the...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
A landscape company is hired to plant trees in three new subdivisions. The company charges the developer for each tree planted, an hourly rate to plant the trees, and a fixed delivery charge. In one...
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
Using any compounds that contain two carbon atoms or fewer, show a way to prepare a racemic mixture of (2R, 3R)- 2,3-dihydroxypentane and (2S,3S)-2,3-dihydroxypentane.
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (g) (h) SH
-
Paclitaxel (marketed under the trade name TaxolTM) is found in the bark of the Pacific yew tree, Taxus berevifolia, and is used in the treatment of cancer: (a) Draw the enantiomer of paclitaxel. (b)...
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...
-
On consolidated financial statements, where does the parents equity in the net income of the subsidiary account appear? A. On the consolidated income statement, as a revenue B. On the consolidated...

Study smarter with the SolutionInn App


