The active ingredient of aspirin tablets is acetylsalicylic acid, which has a density of 1.4 g/cm 3
Question:
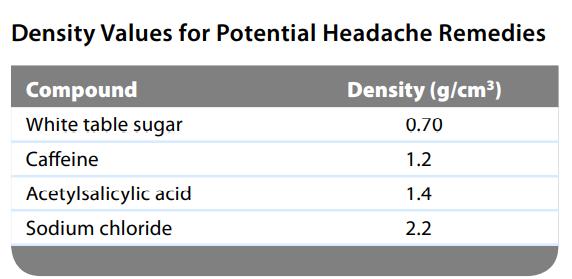
The active ingredient of aspirin tablets is acetylsalicylic acid, which has a density of 1.4 g/cm3. In a lab class, a student used paper chromatography to isolate another common ingredient of headache remedies. The isolated sample had a mass of 0.384 g and a volume of 0.32 cm3. Given the data in the following table, what was the other ingredient in the headache remedy?
Transcribed Image Text:
Density Values for Potential Headache Remedies Density (g/cm³) Compound White table sugar Caffeine Acetylsalicylic acid Sodium chloride 0.70 1.2 1.4 2.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
Other active ingredient found in a headache remedy is iontophylates ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The active ingredient of the insect repellent Off is N, N-diethyl-m-toluamide, m-CH3C6H4CON (CH2CH3)2. Outline a synthesis of this compound starting with 3-methylbenzoic acid (m-toluic acid).
-
Repeat Problem 69 for cobalt, which has a density of 8.9 g/cm3, a molecular mass of 58.9 g/mol, and a saturation magnetization given by 0Ms = 1.79 T.
-
Castor oil, which has a density 0.96 x 103 kg/m 3 at room temperature, is forced through a pipe of circular cross section by a pump that maintains a gauge pressure of 950 pa the pipe has a diameter...
-
General-equilibrium effects with labor complementarity. Consider an economy comprised of 100 cities. Each city initially contains 1 million each of high school dropouts, high school graduates,...
-
Lynette, a famous basketball player, is considering the possibility of transferring the sole right to use her name to promote basketball shoes produced and sold by the NIK Corporation. NIK will pay...
-
Compare the generic UML class diagram for the conversion process shown in Figure 7.5 with a generic sales and collection diagram, similar to Figure 5.9. Identify the similarities and differences and...
-
Role of retailer interest on shopping behavior. Retail interest is defined by marketers as the level of interest a consumer has in a given retail store. Marketing professors investigated the role of...
-
Ten individuals have participated in a diet-modification program to stimulate weight loss. Their weight both before and after participation in the program is shown in the following list is there...
-
Ivanhoe Medical manufactures hospital beds and other institutional furniture. The company's comparative balance sheet and income statement for 2019 and 2020 follow. Ivanhoe Medical Comparative...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
Lipitor, a pharmaceutical drug that has been shown to lower bad cholesterol levels while raising good cholesterol levels in patients taking the drug, had over $3 billion in sales in 2015. Assuming...
-
A 194-g sample of caffeine (C 8 H 10 N 4 O 2 ) contains 6.02 10 23 molecules of caffeine. If a typical 10-hour energy drink contains 422 mg of caffeine, how many molecules of caffeine are present in...
-
Limited liability companies (LLCs) are very popular today as a form of organization. Assume a client asks you to explain what this type of organization is all about. Prepare a brief description of...
-
Design an arithmetic circuit with two selection variables S 1 and S 0 and two n- bit data inputs A and B. The circuit generates the following eight arithmetic operations in conjunction with carry C...
-
Larrys Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Larrys prints...
-
Pecos Pecan Pads makes pressed pecan wood covers to prevent weed growth. During July 2009, the company produced and sold 44,000 rolls and recorded the following cost data: Requirements 1. Compute the...
-
The Human Resources departments costs are allocated to the other departments based on the number of direct labor hours. The departments expected fixed costs are 400,000 and its variable costs are...
-
A lawyer allocates overhead costs based on her hours working with different clients. The lawyer expects to have \($200,000\) in overhead during the year and expects to work on clients cases 2,000...
-
Why are subsidies more likely to be needed for commuter and regional services than for medium-to-long-distance passenger services?
-
What recommendations would you make to Big Four firms to help them (1) avoid confrontations with governmental officials in an authoritarian society and (2) deal effectively with such confrontations...
-
What is the most probable point (not radius) at which a 2p electron will be found in the hydrogen atom?
-
Explicit expressions for hydrogenic orbitals are given in Tables 10.1 and 9.3. (a) Verify both that the 3px orbital is normalized (to I) and that 3px and 3dxy are mutually orthogonal. (b) Determine...
-
Show that l, and 12 both commute with the Hamiltonian for a hydrogen atom. What is the significance of this result?
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


