Use the Gibbs phase rule to determine the number of degrees of freedom in each region of
Question:
Use the Gibbs phase rule to determine the number of degrees of freedom in each region of the phase diagram in Figure 11-6.
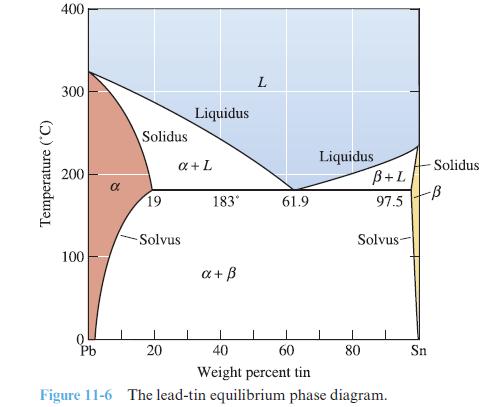
Transcribed Image Text:
Temperature (°C) 400 300 200 100 Pb Solidus 19 -Solvus 20 Liquidus a + L 183° a+ ß L 61.9 Liquidus B+L 97.5 Solvus 80 60 40 Weight percent tin Figure 11-6 The lead-tin equilibrium phase diagram. Solidus -ß Sn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
The Gibbs phase rule is given by F C P 2 where F is the number ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Use the multiplicative rule to determine the number of sample points in the sample space corresponding to the experiment of tossing a coin the following number of times: a. 2 times b. 3 times c. 5...
-
Determine the number of degrees of freedom for a nonadiabatic equilibrium flash for one liquid feed, one vapor stream product, and two immiscible liquid stream products as shown inFigure. V.y, Vapor...
-
Determine the number of degrees of freedom for the two-sample t test or CI in each of the following situations: a. m = 10, n = 10, s1 = 5.0, s2 = 6.0 b. m = 10, n = 15, s1 = 5.0, s2 = 6.0 c. m = 10,...
-
Homzmart was founded in late 2019 by Daraz's former COO Mahmoud Ibrahim and Ibrahim Mohammed who led Jumia's h homzmart logistics development in Egypt to make furniture discovery and shopping easier...
-
(a) Redraw demand curve for pounds D£ and supply curve of pounds S£ as in the figure of Problem 1 and draw on it another supply curve for pounds (label it S*£) that intersects...
-
1. What would you have done if you had been the school administrator receiving calls in this situation? 2. Do you think the school was correct in ignoring the teachers record? 3. Does it make a...
-
Doering Company, a U.S. corporation with customers in several foreign countries, had the following selected transactions for 2013 and 2014. 2013 Pesos (Mexico) ............ $0.1055 Yen (Japan)...
-
Sam Santiago operates a retail variety store. The books include a cash payments journal and an accounts payable ledger. All cash payments (except petty cash) are entered in the cash payments journal....
-
The following income statement and balance sheets for Virtual Gaming Systems are provided. VIRTUAL GAMING SYSTEMS Income Statement For the year ended December 31, 2021 Net Sales $3,001,800 Cost of...
-
Alice J. and Bruce M. Byrd are married taxpayers who file a joint return. Their Social Security numbers are 123-45-6789 and 111-11-1111, respectively. Alice's birthday is September 21, 1966, and...
-
Consider an Al-12% Mg alloy (Figure 11-29). During solidification, determine (a) The composition of the first solid to form; (b) The liquidus temperature, solidus temperature, solvus temperature, and...
-
We discussed the primary phase or primary constituent. Why would we be interested in the percentage of the primary phase in the Al-Si alloy system?
-
A quadratic equation (x) = 0 has a solution x = 2. Its graph has vertex (5, 3). What is the other solution of the equation?
-
Following the example shown in (a) below, indicate the effects of the listed transactions on the assets, liabilities, and stockholders equity of John Dallmus, certified public accountant, a...
-
What effect does the ordering of a search tree have on the efficiency of the search? What effect does it have on the quality of the results? How would order affect the way that depth-first search or...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Sales Revenue Equipment Common Stock Notes Payable Retained Earnings...
-
Smart Sports is also planning to launch a range of drinks products. The products have been developed by Hydration Labs Ltd and are designed to be sold as powders that dissolve easily in water. They...
-
Baucom Company accepted credit cards in payment for \(\$ 6,850\) of services performed during March 2011. The credit card company charged Baucom a 4 percent service fee. The credit card company paid...
-
On April 1, 2013, Abbots acquired all the issued common shares (cum div.) of Evion for $100,000. At that date, relevant balances in the records of Evion were: Share capital .. $80,000 Retained...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
Identify the Lewis acids in the following reactions: a. b. BF3 + NH3 FB-NH3
-
Gaseous SO 2 is created by combustion of sulfur-containing fuels, especially coal. Explain how O 2 in the atmosphere makes acidic rain.
-
Identify the Brnsted-Lowry acids among the reactants in the following reactions: a. b. KCN + HI = HCN + KI
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...

Study smarter with the SolutionInn App


