Use the Lewis acidbase model to explain the following reaction. CO(g) + HO(1) HCO3(aq)
Question:
Use the Lewis acid–base model to explain the following reaction.
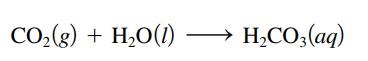
Transcribed Image Text:
CO₂(g) + H₂O(1) H₂CO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The Lewis acidbase model explains chemical reactions in terms of the transfer of electrons from a Le...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Propose a mechanism to explain the following reaction by using words, and in doing so predict the absolute stereochemistry (R or S) at the stereocentre in the product. Then draw three other product...
-
Use Lewis symbols to show the reaction of atoms to form arsine, AsH3. Indicate which electron pairs in the Lewis formula of AsH3 are bonding and which are lone pairs.
-
The following are representations of acid base reactions: a. Label each of the species in both equations as an acid or a base and explain. b. For those species that are acids, which labels apply:...
-
At year-end 2010, 28,879 million represents: A. the funded status of the plan. B. the defined benefit obligation. C. the fair value of the plans assets. Kensington plc, a hypothetical company based...
-
Explain the four major principles of risk classification.
-
How is network performance measured?
-
SEC filings of publicly traded companies are available to view online. LO5 Address: http//biz.yahoo.com/i Steps 1. Pick a company and type in the companys name. 2. Choose Quote. Instructions Answer...
-
Archer Foods has a freezer that is in need of repair and is considering whether to replace the old freezer with a new freezer or have the old freezer extensively repaired. Information about the two...
-
Samsung Co. just paid a dividend of $4.50. The dividend is paid on annual basis. The required annual rate of return is 12 percent. If the dividend is expected to grow at 4 percent every year, what...
-
The file azcounties.dat gives data from the 2000 U.S. Census on population and housing unit counts for the counties in Arizona (excluding Maricopa County and Pima County, which are much larger than...
-
Is an aqueous solution of NaHSO 4 acidic, basic, or neutral? What reaction occurs with water? Calculate the pH of a 0.10-M solution of NaHSO 4 .
-
Calculate the pH of a 0.010-M solution of iodic acid (HIO 3 , K a = 0.17).
-
Consider the following horizontal box plot: a. What is the median of the data set (approximately)? b. What are the upper and lower quartiles of the data set (approximately)? c. What is the inter...
-
Which of the five hazardous attitudes do you display most frequently? What can you do to minimize the presence and impact of these attitudes in your life?
-
What brought you to this course? How do you define Black or Blackness? What do you hope to get out of this class? When you think of Black Culture, what is the first thing that comes to mind? [For...
-
1. What is XBRL Taxonomy? How do you as a preparer of financial statement use the XBRL Taxonomy in locating a label for a specific financial element? 2. What are the benefits of adopting XBRL from...
-
What a business can do to protect and minimize the invasion of privacy for their customers? Think of your experience when visiting a website. What do most websites have you agree to before you do...
-
Based on your interest, skill set, or goals what do you typically contribute when working in groups? What do you need others to contribute due to your lack of interest, skill set, or goals? How do...
-
A state trooper is hidden 30 feet from a highway. One second after a truck passes, the angle between the highway and the line of observation from the patrol car to the truck is measured. See the...
-
Three successive resonance frequencies in an organ pipe are 1310, 1834, and 2358 Hz. (a) Is the pipe closed at one end or open at both ends? (b) What is the fundamental frequency? (c) What is the...
-
The hydrolysis of a biological thioester to the corresponding carboxylate is often more complex than the overall result might suggest. The conversion of succinyl CoA to succinate in the citric add...
-
One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehydes 3-phosphate. The process occurs by phosphorylation with...
-
Penicillins and other fl-lactam antibiotics (see the Focus On in this chapter) typically develop a resistance to bacteria due to bacterial synthesis of -lactamase enzymes. Tazobactam, however, is...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost
-
For esch of the following Independent tranactiona, determine the minimum amount of net income or loas for tox purposes snd the tsxpsyer to which it applies. 1 An individual purchases a $ 1 0 , 0 0 0...

Study smarter with the SolutionInn App


