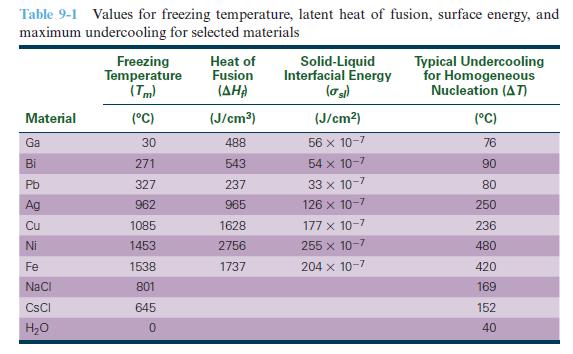
Using the densities in Appendix A, convert the heats of fusion in Table 9-1 from units of
Question:
Using the densities in Appendix A, convert the heats of fusion in Table 9-1 from units of J/cm3 to kJ/kg.
Transcribed Image Text:
Table 9-1 Values for freezing temperature, latent heat of fusion, surface energy, and maximum undercooling for selected materials Material Ga Bi Pb Ag 23 22 Cu Ni Fe NaCl CsCl H₂O Freezing Temperature (Tm) (°C) 30 271 327 962 1085 1453 1538 801 645 0 Heat of Fusion (AHA) (J/cm³) 488 543 237 965 1628 2756 1737 Solid-Liquid Interfacial Energy (σ sl) (J/cm²) 56 x 10-7 54 x 10-7 33 x 10-7 126 x 10-7 177 x 10-7 255 x 10-7 204 x 10-7 Typical Undercooling for Homogeneous Nucleation (AT) (°C) 76 90 80 250 236 480 420 169 152 40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
QUESTION 1 Aluminum has a density of 1.35 g/cm. What is the mass of a rectangular block of aluminum measuring 11.1 cm by 22.2 cm by 34.6 cm? O a. none of these Ob.0.159 kg O c. 11.5 kg O d. 183 kg...
-
Calculate the acceleration of the center of mass of the system of the four 10-kg cylinders. Neglect friction and the mass of the pulleys and cables. 500 N 250 N 10 10 kg kg 10 kg 10 kg
-
Using the heats of fusion and vaporization for water, calculate the change in enthalpy for the sublimation of water: H2O(s) H2O(g) Using the H value given in Exercise 24 and the number of hydrogen...
-
You are considering an investment that will be valued at $1,000 one year from now. Your next best alternative investment opportunity would yield an annual rate of return of 8%, In other words, if you...
-
In April 2000, the seasonally adjusted unemployment rate was 3.8 percent. By June 2001, the unemployment rate had increased to 4.5 percent. Yet the measures by the Federal Reserve to reduce...
-
Futures contracts on stock indices are very popular. Why do you think that is so? How do you think they might be used?
-
Where do we get the amounts to enter in the Unadjusted Thai Balance columns of a work sheet?
-
Consider the following transactional data for the first month of operations for Shine King Cleaning. Nov. 1 Stockholders contributed $ 35,000 and a truck, with a market value of $ 8,000, to the...
-
On December 31, 2020, Carla Co. performed environmental consulting services for Hayduke Co. Hayduke was short of cash, and Carla Co. agreed to accept a $318,100 zero-interest-bearing note due...
-
1. Based on the information presented in this case, how would you describe Shake Shacks retail image? 2. How would you describe the store atmosphere that Shake Shack seeks to maintain? 3. The...
-
Explain the meaning of each term in Equation 9-2. 20. Tm , (9-2)
-
What is the difference between homogenous nucleation and heterogeneous nucleation?
-
The Johari Window emphasizes that we may not be aware of everything that there is to know about ourselves. Others may know things about us that we just cannot see or are unwilling to admit. One way...
-
On January 1, 20X1, Popular Creek Corporation organized SunTime Company as a subsidiary in Switzerland with an initial investment cost of Swiss francs (SFr) 76,000. SunTime's December 31, 20X1, trial...
-
In November 2 0 2 4 , Lily informed you that she needs additional cash flow to meet her personal debt obligations. Lily does not want to sell more stock than she needs to because she wants...
-
Bennett limited provides mobile library services to the community of longbourn. bennett has preliminary operating results for the first year and the company found that net income is different from...
-
What is printed when the value of x is 34? if (x < 32 ) { if (x22) { } System.out.println("Blue"); else if (x <10) { } System.out.println("Red"); else if (x > 25 ) { System.out.println("Yellow"); } }...
-
Heidi expresses concern in the video that when employees are also friends, holding them accountable for performance "doesn't come as naturally" to her. She asks you, "Does my focus on relationship...
-
(a) If u and v have the same magnitude, show that u + v and u - v are orthogonal. (b) Use this to prove that an angle inscribed in a semicircle is a right angle (see the figure). -V
-
5. Convert the following ERD to a relational model. SEATING RTABLE Seating ID Nbr of Guests Start TimeDate End TimeDate RTable Nbr RTable Nbr of Seats RTable Rating Uses EMPLOYEE Employee ID Emp...
-
Which of the following mixtures would result in a buffered solution when 1.0 L of each of the two solutions are mixed? a. 0.2 M HNO 3 and 0.4 M NaNO 3 b. 0.2 M HNO 3 and 0.4 M HF c. 0.2 M HNO 3 and...
-
Which of the following mixtures would result in buffered solutions when 1.0 L of each of the two solutions are mixed? a. 0.1 M KOH and 0.1 M CH 3 NH 3 Cl b. 0.1 M KOH and 0.2 M CH 3 NH 2 c. 0.2 M KOH...
-
Calculate the pH of a solution that is 0.20 M HOCl and 0.90 M KOCl. In order for this buffer to have pH = pK a , would you add HCl or NaOH? What quantity (moles) of which reagent would you add to 1.0...
-
Case Products manufactures two models of DVD storage cases: regular and deluxe. Presented is standard cost information for each model: Cost Components Regular Deluxe Direct materials Lumber 2 board...
-
A corporate bond that you own at the beginning of the year is worth $930. During the year, it pays $56 in interest payments and ends the year valued at $920. What was your dollar return and percent...
-
Anissa makes custom bird houses in her garage and she buys all her supplies from a local lumber yard. Last year she purchased $4500 worth of supplies and produced 2500 bird houses. She sold all 2500...

Study smarter with the SolutionInn App


