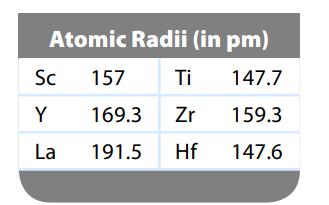
We expect the atomic radius to increase going down a group in the periodic table. Can you
Question:
We expect the atomic radius to increase going down a group in the periodic table. Can you suggest why the atomic radius of hafnium breaks this rule? (See data below.)
Transcribed Image Text:
Atomic Radii (in pm) 147.7 159.3 147.6 Sc Y La 157 169.3 191.5 Ti Zr Hf
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
To what group in the periodic table would an element with atomic number 114 belong?
-
To what group in the periodic table would an element with atomic number 114 belong?
-
The changes in electron affinity as one goes down a group in the periodic table are not nearly as large as the variations in ionization energies. Why?
-
Featherstone Inc. reported the following data: Net income ................................... $296,000 Depreciation expense ................... 113,100 Gain on disposal of equipment ...... 58,500...
-
Valencia Products makes automobile radar detectors and assembles two models: LaserStop and SpeedBuster. The firm can sell all it produces. Both models use the same electronic components. Two of these...
-
What are the electric fields at points 1, 2, and 3 in FIGURE P22.64? Give your answer in component form. 2.0 cm 5.0 nC +. 1.0 cm 2.0 cm FIGURE P22.64
-
Jones expects an immediate investment of $57,466 to return $10,000 annually for eight years, with the first payment to be received one year from now. What rate of interest must Jones earn? (Use Table...
-
For 2008, Orchard Corporation reported after-tax net income of $5,800,000. During the year, the number of shares of stock outstanding remained constant at 10,000 of $100 par, 9 percent preferred...
-
Herold filed his 2018 return on June 21st 2019. He had a valid extension. Under original circumstances, which is the last day he can foul and amended 2018 return to receive a refund of text paid?
-
A plastic injection molding machine operates 348 days a year. It is the bottleneck workstation in a process that produces plastic housed wall clocks. Industrial engineers collected data on the...
-
Calculate, to four significant figures, the longest and shortest wavelengths of light emitted by electrons in the hydrogen atom that begin in the n = 5 state and then fall to states with smaller...
-
The four most abundant elements by mass in the human body are oxygen, carbon, hydrogen, and nitrogen. These four elements make up about 96% of the human body. The next four most abundant elements are...
-
Evaluate the integral. sin* (31) dr dt
-
From your reading this unit on motivation and change from the TIP series, what is the connection and interplay between these concepts/statements below in your opinion in working with clients facing...
-
Please help with the following The partnership of Bauer, Ohtani, and Souza has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 276,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Define meaning of partnership deed.
-
List down the information contains in the partnership deed.
-
A Norman window has the shape of a rectangle surmounted by a semicircle. (Thus the diameter of the semicircle is equal to the width of the rectangle. See Exercise 1.1.62.) If the perimeter of the...
-
Explain what is meant by vicarious liability and when it is available?
-
The systematic name of the ? CH = CH2 group is ethenyl: Provide a systematic name for limonene, which is found in lemons and other citrus-fruits. Limonene
-
Vitamin A alcohol is 3, 7 dimethyl-9-(2, 6, 6-trimethyl-1-cyclohexenyl)-2, 4, 6, 8-nonatetraen-1-o1, Draw the stricter of vitamin A alcohol.
-
Explain which compound has the higher melting point: (a) Cyclopentane or pentane (b) 1-Pentanol or pentane
-
A person purchased a $181,873 home 10 years ago by paying 20% down and signing a 30-year mortgage at 8.4% compounded monthly. Interest rates have dropped and the owner wants to refinance the unpaid...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...

Study smarter with the SolutionInn App


