What type of molecular orbital would result from the in-phase combination of two d xz atomic orbitals
Question:
What type of molecular orbital would result from the in-phase combination of two dxz atomic orbitals shown below? Assume the x-axis is the internuclear axis.
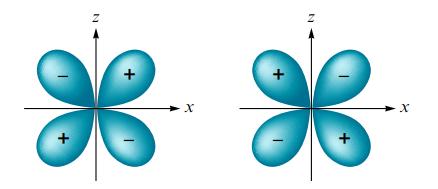
Transcribed Image Text:
z Z X X X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
When the two electrons come together and they are out of ...View the full answer

Answered By

Hardik Dudhat
I am semi-qualified Chemical Engineering ,I have scored centum in accounting in my senior secondary and in my graduation. I have always helped my fellow students with their concerns on the subject, i have tutored on various tutoring sites in the past and also have taken home tuitions for degree and MBA students. As a tutor, I don't want my students to just get a solution, I want them to understand the concept and never have a doubt in that area thereon and i believe in excelling and not in educating.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The following sketches show the atomic orbital wave functions (with phases) used to construct some of the MOs of a homo-nuclear diatomic molecule. For each sketch, determine the type of MO that will...
-
The sp2 hybrid atomic orbitals have the following general form: where Ïs, Ïpx, and Ïpy represent orthonormal (normalized and orthogonalized) atomic orbitals. Calculate the values of A...
-
Assume that the cyanide ion, CN, has molecular orbitals similar to those of a homonuclear diatomic molecule. Write the configuration and bond order of CN. Is a substance of the ion diamagnetic or...
-
Roles define the manager. Do you agree or disagree with this statement? Discuss what you think managers do.
-
Why do firms divest assets?
-
The Town of Willingdon adopted the following General Fund budget for the fiscal year beginning July 1: Estimated revenues: Taxes...
-
ALTERNATIVE DIVIDEND POLICIES In 2013, Keenan Company paid dividends totaling $3,600,000 on net income of $10.8 million. Note that 2013 was a normal year and that for the past 10 years, earnings have...
-
The December 31, 2014, unadjusted trial balance for Musical Sensations after its second year of operations follows: Required 1. Enter the unadjusted trial balance onto a work sheet. 2. Using the...
-
PLEASE HELP. Fiona Starr (social security number 111-22-3333) is single and earns $105,000 as a manager at Costco. Three years ago, she bought a single-family home on 12 Beach St., Bangor, ME 55555,...
-
The 2014-T6 aluminium rod has a diameter of 30 mm and supports the load shown. Determine the displacement of end A with respect to end E. Neglect the size of the couplings. 8 kN 2 kN 6 kN 4 kNY -2m-...
-
Using molecular orbital theory, explain why the removal of an electron from O 2 strengthens bonding, whereas the removal of an electron from N 2 weakens bonding.
-
The transport of O 2 in the blood is carried out by hemoglobin. Carbon monoxide (CO) can interfere with O 2 transport because hemoglobin has a stronger affinity for CO than for O 2 . If CO is...
-
Condensing steam at 150oC is used on the inside of a bank of tubes to heat a cross-flow stream of CO2 that enters at 3 atm, 35oC, and 5 m/s. The tube bank consists of 100 tubes of 1.25-cm OD in a...
-
Caching. CPUs. Hard disk drives. Parallelism. Pipelining. RAM. Registers. Solid-state drives. Dinkleberg 1. (3 points) You know that neighbor who always one-ups you? Apparently, he just bought a...
-
Information for Question 1 and 2: The National Health Interview Survey is an annual national health survey of people living in about 35,000 randomly selected households in the United States. On the...
-
Q1. There is trampoline on the ground, and connected to spring of spring constant, k, on the bottom. You are holding a child of mass, M on a H tall building top (H is measured from the trampoline to...
-
SPSS for intermediate statistics extra problems solutions d. Approximately how much variance in consumers' tendency to purchase the product could be predicted from these variables? If you owned the...
-
A finance company uses the discount method of calculating interest. The loan principal is $5,000, the interest rate is 10%, and repayment is expected in two years. You will receive 4196.25 X from the...
-
1. Even though Sullivan supplied a physicians certification, did the agency have the right to require a second medical evaluation? 2. How could Sullivan have avoided the conflict and qualified for...
-
Cassandra Casey operates the Futuristic Antique Store. She maintains subsidiary ledgers for accounts payable and accounts receivable. She presents you with the following information for October 2019:...
-
What is the difference between a transition state and an intermediate?
-
Draw an energy diagram for a one-step reaction with Keq < 1. Label the parts of the diagram corresponding to reactants, products, transition state, G, and G++. Is G positive or negative?
-
Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall G, transition states, and intermediate. Is G positive or negative?
-
Use the following information: \ table [ [ Country , \ table [ [ Consumer Prices ] ] , Interest Rates,Current Units ( per US$ ) ] , [ Forecast , 3 - month, 1 - yx Covt Bond,, ] , [ 2 0 2 4 e ,...
-
Year-to-date, Yum Brands had earned a 3.70 percent return. During the same time period, Raytheon earned 4.58 percent and Coca-Cola earned 0.53 percent. If you have a portfolio made up of 40 percent...
-
Rate of Return If State Occurs State of Probability of Economy State of Economy Stock A Stock B Stock C Boom .15 .31 .41 .21 Good .60 .16 .12 .10 Poor .20 .03 .06 .04 Bust .05 .11 .16 .08 a. Your...
Invisible Influence The Hidden Forces That Shape Behavior 1st Edition - ISBN: 1476759731 - Free Book

Study smarter with the SolutionInn App


