A gas mixture contains 2 kg O 2 and 5 kg N 2 . For this mixture,
Question:
A gas mixture contains 2 kg O2 and 5 kg N2. For this mixture, determine the total volume at 300 K and 101 kPa (atmospheric conditions) in two different ways, as given in parts A and B below:
A. Consider the oxygen and nitrogen as being in separate chambers.
B. Find the apparent molecular weight,Mmix, and gas constant, Rmix, for the mixture. Use Rmix in the ideal gas law to find the volume of the mixture.
C. Compare the total volumes that you found in parts a and b. Why do both methods give the same answer?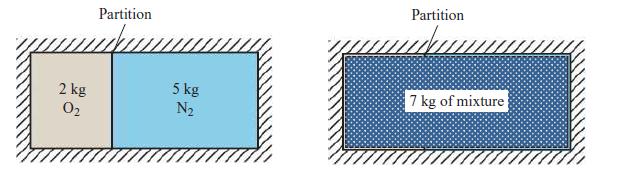
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





