A rigid tank contains 5 kg of O 2 , 8 kg of N 2 , and
Question:
A rigid tank contains 5 kg of O2, 8 kg of N2, and 10 kg of CO2 at 100 kPa and 1000 K. Assume that the mixture behaves as an ideal gas. Determine
(a) The mass fraction of each component,
(b) The mole fraction of each component,
(c) The partial pressure of each component,
(d) The average molar mass (apparent molecular weight) and the gas constant of the mixture,
(e) The volume of the mixture in m3.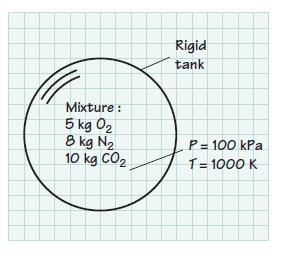
Transcribed Image Text:
Mixture : 5 kg 0₂ 8 kg N₂ 10 kg CO₂ Rigid tank P = 100 kPa T = 1000 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Given data Mass of O2 5 kg Mass of N2 8 kg Mass of CO2 10 kg Total mass of mixture 23 kg Pressure P ...View the full answer

Answered By

Joshua Marie Geuvara
I am an academic writer with over 5 years of experience. I write term papers, essays, dissertations, reports, and any other academic paper. My main objective is to produce a high-quality paper free from plagiarism and ensure a student scores an A+. Being a fluent English speaker, I have great communication skills that also enable me to produce excellent papers.
I am conversant with most academic referencing styles (APA, MLA, and Harvard).
You can trust me with your paper and expect nothing less than quality and excellent results. I look forward to meeting with you and, more importantly, developing something that will both make us happy and satisfied.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
A rigid tank contains 5 kg of saturated vapor steam at 100C. The steam is cooled to the ambient temperature of 25C. (a) Sketch the process with respect to the saturation lines on a T-v diagram. (b)...
-
A rigid tank contains 5 kg of ethylene at 3 MPa, 30C. It is cooled until the ethylene reaches the saturated vapor curve. What is the final temperature?
-
A rigid tank contains 5 kg of ethylene at 3 MPa, 30C. It is cooled until the ethylene reaches the saturated vapor curve. What is the final temperature?
-
9. A molybdenum-vanadium alloy of composition 50wt%Mo - 50wt%V is slowly cooled from a temperature of 2600C to 1800C. Determine: a) At what temperature does the first solid phase form? b) What is the...
-
Barry Means and his spouse Mary want to transfer their one- third (1/ 3) stock interest in The Diamond Ridge Golf Course to a trust for the benefit of their two children. The couples CPA has advised...
-
Research Problem 3. Ashby and Curtis, married professionals, have a 2-year-old son, Jason. Curtis works full-time as an electrical engineer, but Ashby has not worked outside the home since Jason was...
-
What are the conditions that define g, the long-term growth rate of core earnings used in the Gordon Model?
-
The compensation committee of a large public corporation engages you to help design a tax efficient compensation plan for the current chief executive officer (CEO). In a preliminary interview with...
-
a) Explain the importance of portfolio performance evaluation in investment analysis. b) Explain the major component activities of portfolio performance evaluation
-
In one of PLEs manufacturing facilities, a drill press that has three drill bits is used to fabricate metal parts. Drill bits break occasionally and need to be replaced. The present policy is to...
-
A 3-ft 3 rigid vessel contains a 5050 mixture of N 2 and CO (by volume). Determine the mass of each component for T = 65 F and P = 30 psia.
-
An instrument for the analysis of trace hydrocarbons in air, or in the products of combustion, uses a flame ionization detector. The flame in this device is fueled by a mixture of 40% (vol.) hydrogen...
-
Distinguish between flexible budgets and master (static) budgets.
-
Consider the following thermochemical equation: 2 Na 2 O 2 (s) + 2 H 2 O(l) 4 NaOH(s) + O 2 (g) H = -126 kJ Calculate the enthalpy change when 41.5 g of Na 2 O 2 with water?
-
Newton's Laws Introduction Problems
-
Mr. A and B agreed to start a business agreed to share profit and loss based the condition that will profit only when there is profit in excess of BD 10,000 this from of business is called as:...
-
L= {a'e"b"d' | i=1+m and l,m,n 20] a. Write at least 10 strings of the above language in increasing order of string length. b. Write Context Free Grammar (CFG) for the above language.
-
The Beta Co. shows the following results of operation on Dec. 31. Variable cost Fixed costs Direct materials P512,500 Direct labor 575,000 Manufacturing overhead 400,000 P212,500 For the year then...
-
There is an increasing trend toward using crowd sourcing as a research and development tool. According to Gartner, by 2017 around 75 percent of all product development capabilities carried out by...
-
We all experience emotions, but some people disguise their true feelings better than others. Do you think this is a helpful or harmful thing to do? Under what conditions do you think it would be most...
-
Repeat exercise 19 for the combustion of coal. For simplicity assume coal is just carbon, C, with a HV of 32,800 kJ/kg. Use engineering considerations to give insight on the global warming issue....
-
Given the heat of formation of liquid methanol, CH 3 OH(l), is 238,000 kJ/kmol, what is its heat of combustion in kJ/kg?
-
In 1800 the main fuel used in the United States was wood. (Assume wood is cellulose, whose representative repeating formula is C 6 H 12 O 6 .) In 1900, the main fuel used was coal (assume coal in...
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...

Study smarter with the SolutionInn App


