A0.6-m 3 tank contains 0.2 kg of H 2 O at 350 K. Determine the pressure in
Question:
A 0.6-m3 tank contains 0.2 kg of H2O at 350 K. Determine the pressure in the tank and the enthalpy of the H2O.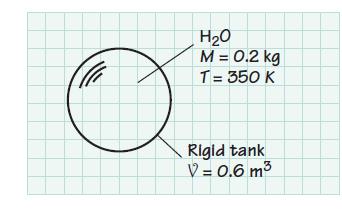
Transcribed Image Text:
H₂O M = 0.2 kg T= 350 K Of Rigid tank V = 0.6 m³
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the pressure in the tank we can use the ideal gas law which states that PV nRT where P ...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
A rigid tank of volume 83 m3 contains 100 kg of H2O at 100oC. The tank is heated until the temperature inside reaches 120oC. Determine the pressure (p) inside the tank at (a) The beginning. (b) The...
-
A tank contains 5 kg of saturated liquid and 5 kg of saturated vapor of H2O at 500 kPa. Determine (a) Its volume in m3 an (b) Its temperature (T) in Celcius. The tank is now heated to a temperature...
-
A rigid tank contains 1 kg of H2O at 100 kPa, x = 0.1. Given that the tank can withstand a maximum internal pressure of 5 MPa, determine (a) The maximum temperature to which the steam in the tank can...
-
What conditions must be met for revenue to be recorded? Can pledges meet those conditions?
-
Assume now that Office Helpers decides to go public and would like to offer its shares at a target price of $10 per share. If the IPO is likely to be underpriced by 20%, how many shares should the...
-
Comment on the costs that Joe would incur from each of these two errors. Which of the two errors would be more costly? Which of the two outcomes (winning or losing the suit) is more likely to occur?...
-
A part processed in a flexible manufacturing system (FMS) is routed through a set of operations, some of which are sequential and some of which are parallel. In addition, an FMS operation can be...
-
Information on Canyon Chemicals direct materials costs follows: Quantities of chemical Y purchased and used . . . . . 28,800 gallons Actual cost of chemical Y used . . . . . . . . . . . . . . ....
-
Carla Vista Leasing Company leases a new machine to Sandhill Corporation. The machine has a cost of $ 6 5 , 0 0 0 and fair value of $ 8 9 , 0 0 0 . Under the 3 - year, non - cancelable contract,...
-
You work in the human resources department of your company helping new employees fill out the necessary paperwork to get their first paycheck. There are a number of decisions that employees must make...
-
Wet steam exits a turbine at 50 kPa with quality 0.83. Determine the following properties of the wet steam: T, v, h, u, and s. Give the units. Steam in Steam turbine Steam out Pexit = 50 kPa Xexit =...
-
Steam is condensing in the shell of a heat exchanger at 305 K under steady conditions. The volume of the shell is 2.75m 3 . Determine the mass of the liquid in the shell if the specific enthalpy of...
-
A typical nuclear reactor produces about 1.0 MW of power per day. What is the minimum rate of mass loss required to produce this much energy?
-
Q10: Region ( experienced compressive stresses and has a than the rest of the bracket. Region ( ) experienced tension stresses and has a of the bracket. Deep Drawing and Stretch Forming width (into...
-
A sample of 1500 computer chips revealed that 32% of the chips do not fail in the first 1000 hours of their use. The company\'s promotional literature claimed that above 29% do not fail in the first...
-
The 75 lb block is released from rest 5 ft above the plate. Determine the compression of each spring when the block momentarily comes to rest after striking the plate. Neglect the mass of the plate....
-
Indiana Soy Products (OSP) buys soybeans and processes them into other soy products. Each ton of soybeans that OSP purchases for $250 can be converted for an additional $180 into 675 lbs of soy meal...
-
The 2025 Annual Report of Splish International contains the following informatio (in millions) June 29, 2025 June 27, 2024 Total assets $1,545 $1,502 Total liabilities 989 1,060 Net sales 2,800 2.971...
-
Why is parallel conversion costly?
-
KD Insurance Company specializes in term life insurance contracts. Cash collection experience shows that 20 percent of billed premiums are collected in the month before they are due, 60 percent are...
-
An inventor claims to have designed a heat engine that receives 120 Btu of heat and generates 30 Btu of useful work when operating between a high-temperature energy reservoir at 140F and a...
-
A person can blink an eye in approximately 7 ms. At what speed (in revolutions per minute) would a four-stroke engine be operating if its power stroke took place literally in the blink of an eye? Is...
-
A four-stroke gasoline engine produces an output of 35 kW. Using the density of gasoline listed in Table 6.1, the heating value for gasoline in Table 7.3, and a typical efficiency listed in Table...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...

Study smarter with the SolutionInn App


