Apply interpolation to the property data in Table B.3 to determine the following properties of superheated steam:
Question:
Apply interpolation to the property data in Table B.3 to determine the following properties of superheated steam:
A. The specific volume at 523 K and 1.0 MPa
B. The mass-specific enthalpy at 815 K and 0.3 MPa
C. The mass-specific internal energy at 620 K and 4.5 MPa
Table B.3
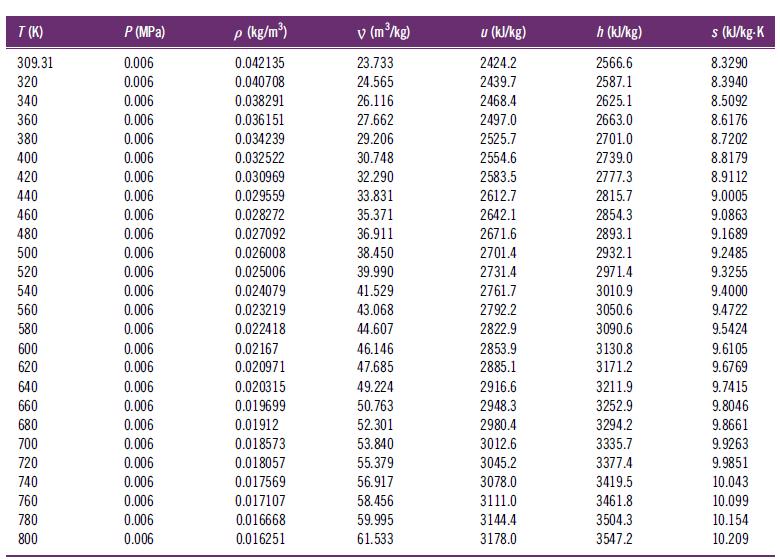
Transcribed Image Text:
T (K) 309.31 320 340 360 380 400 420 440 460 480 500 520 540 560 580 600 620 640 660 680 700 720 740 760 780 800 P (MPa) 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 0.006 p (kg/m³) 0.042135 0.040708 0.038291 0.036151 0.034239 0.032522 0.030969 0.029559 0.028272 0.027092 0.026008 0.025006 0.024079 0.023219 0.022418 0.02167 0.020971 0.020315 0.019699 0.01912 0.018573 0.018057 0.017569 0.017107 0.016668 0.016251 v (m³/kg) 23.733 24.565 26.116 27.662 29.206 30.748 32.290 33.831 35.371 36.911 38.450 39.990 41.529 43.068 44.607 46.146 47.685 49.224 50.763 52.301 53.840 55.379 56.917 58.456 59.995 61.533 u (kJ/kg) 2424.2 2439.7 2468.4 2497.0 2525.7 2554.6 2583.5 2612.7 2642.1 2671.6 2701.4 2731.4 2761.7 2792.2 2822.9 2853.9 2885.1 2916.6 2948.3 2980.4 3012.6 3045.2 3078.0 3111.0 3144.4 3178.0 h (kJ/kg) 2566.6 2587.1 2625.1 2663.0 2701.0 2739.0 2777.3 2815.7 2854.3 2893.1 2932.1 2971.4 3010.9 3050.6 3090.6 3130.8 3171.2 3211.9 3252.9 3294.2 3335.7 3377.4 3419.5 3461.8 3504.3 3547.2 s (kJ/kg-K 8.3290 8.3940 8.5092 8.6176 8.7202 8.8179 8.9112 9.0005 9.0863 9.1689 9.2485 9.3255 9.4000 9.4722 9.5424 9.6105 9.6769 9.7415 9.8046 9.8661 9.9263 9.9851 10.043 10.099 10.154 10.209
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
Apply interpolation to find the following properties of water from Table B3 A B C Specif...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Apply interpolation to the property data in Table B.3 to determine the following properties of superheated steam: A. The specific volume at 727 K and 1.5 MPaB. The mass-specific enthalpy at 823 K and...
-
Apply interpolation to the property data in Table B.3 to determine the following properties of superheated steam: A. The specific volume at 637 K and 700 kPaB. The mass-specific enthalpy at 752 K and...
-
How to Interpolate. Apply interpolation to the property data in Table B.2 to determine the following properties of saturated vapor H 2 O:A. The specific volume at 50.8 kPa.B. The saturation...
-
The net present value and internal rate of return desirability measures for two mutually exclusive investments being considered by Stockton Corporation to follow. Year NPV IRR R 161 14.60% S 138...
-
Now assume that Plastico is considering a project that requires an initial investment of $100 million and has the following projected income statement (depreciation for the project is expected to be...
-
When a television network buys the rights to show a series for film, the cost of the rights are treated as assets on the balance sheet as program rights. Several years ago ABCs practice was to...
-
Review some major influences on our current thinking about performance? lo1
-
Weinberg Canning produces fillet, smoked salmon, and salmon remnants in a single process. The same amount of disposal cost is incurred whether a product is sold at split-off or after further...
-
A company has the following balances for the year ended 30th June 2020: cost of goods sold $340,000, beginning inventory $20,000, sales $691,000, sales returns $20,000 and cost of goods available for...
-
Lunatics, an e-commerce sports company wants to buy Rowdy Trading Cards at a cost of $504 million. Rowdy will operate for 20 years. They expect annual cash flows from operations to be $70.1 million...
-
Steam enters the condenser of a modern power plant with temperature 32 C and quality 0.98. The condensate (water) leaves at 7 kPa and 27 C. Determine the change in specific volume between the inlet...
-
Water at 3.4 MPa is pumped through pipes embedded in the concrete of a large dam. The water, in picking up the heat of hydration of the concrete curing, increases in temperature from 10 C to 40 C....
-
Why are accruals called spontaneous sources of funds, what are their costs, and why dont firms use more of them? AppendixL01
-
PP Company purchases a material that is then processed to yield three chemicals: anarol, estyl, and betryl.In June, PPC purchased 10,000 gallons of the material at a cost of $250,000, and the company...
-
Suppose Boyson Inc. free cash flow for the next year is $ 1 5 0 , 0 0 0 and the FCF is expected to grow a concert rate of 6 . 5 % if WACC is 1 2 . 5 % what is the market value of the firm?
-
An eight lane urban freeway (four lanes in each direction) is on rolling terrain and has 11-ft lanes with a 4-ft right-side shoulder. The interchange density is 1.25 per mile. The base free-flow...
-
For the following business events, please indicate the increase (+) or decrease (-) on the following income statement and balance sheet categories. If there is no effect, leave the box blank. If...
-
4. Change the magnet to the original orientation and drag through the coil. a. What happens to the voltage and light bulb as the North Pole moves through the coil? b. What happens to the voltage and...
-
Which company is todays top seller of ERP systems in the United States?
-
Kims Konstructions has assembled the following data for a proposed straw-reinforced brick maker (SRBM): SRBM Cost: $26,000 Life: 5 years Revenue (p.a.) $11,000 Operating Expenses (p.a.) $3,000...
-
(a) A 1.5-mm-diameter steel sphere (7830 kg/m 3 ) is dropped into a tank of SAE 30 oil. What is its terminal velocity? (b) If the sphere is instead dropped into a different oil of the same density...
-
A 175-lb skydiver reaches a terminal velocity of 150 mph during free fall. If the frontal area of the diver is 8 ft 2 , what are: (a) The magnitude of the drag force acting on the skydiver? (b) The...
-
A 14-mm-diameter sphere is dropped into a beaker of SAE 30 oil. Over a portion of its downward motion, the sphere is measured to fall at 2 m/s. In the units of newtons, what is the drag force acting...
-
Calculate Social Security taxes, Medicare taxes and FIT for Jordon Barrett. He earns a monthly salary of $11,100. He is single and claims 1 deduction. Before this payroll, Barretts cumulative...
-
Bass Accounting Services expects its accountants to work a total of 26,000 direct labor hours per year. The company's estimated total indirect costs are $ 260,000. The company uses direct labor hours...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....

Study smarter with the SolutionInn App


