Consider 0.25 kg of steam contained in a rigid container at 600 K and 4MPa. The steam
Question:
Consider 0.25 kg of steam contained in a rigid container at 600 K and 4MPa. The steam is cooled to 300 K. Determine the entropy change of the H2O associated with this cooling process. Note: Find S2 – S1, not s2 – s1.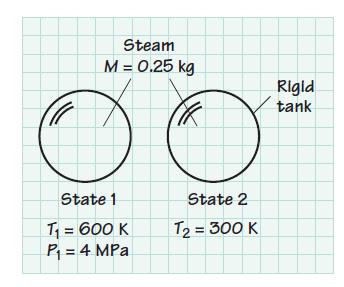
Transcribed Image Text:
Steam M = 0.25 kg State 1 T₁ = 600 K P₁ = 4 MPa State 2 T₂ = 300 K Rigid tank
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
To determine the entropy change of the steam we can use the formula S S2 S1 dqT where S is the ...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
A 250-L rigid tank contains methane gas at 500C, 600 kPa. The tank is cooled to 300 K. a. Find the final pressure and the heat transfer for the process. b. What is the percent error in the...
-
A constant-volume tank contains 5 kg of air at 100 kPa and 327C. The air is cooled to the surroundings temperature of 27C. Assume constant specific heats at 300 K. (a) Determine the entropy change of...
-
The mass of air contained in a rigid 20-L volume at 3 MPa with a temperature of 130 K is nearest: (A) 2.7 kg (B) 2.1 kg (C) 1.8 kg (D) 1.5 kg
-
Under which of the following circumstances would an auditor be most likely to intensify an challenging examination of a $500 imprest petty cash fund a. Reimbursement occurs twice each week. b. The...
-
Pfizer, one of the largest pharmaceutical companies in the United States, is considering what its debt capacity is. In March 1995, Pfizer had an outstanding market value of equity of $24.27 billion,...
-
$8,000 received at the beginning of each year for ten years, compounded at a 6 percent annual rate.
-
Consider the population described by the probability distribution shown below. The random variable x is observed twice. If these observations are independent, verify that the different samples of...
-
During the last few years, Jana Industries has been too constrained by the high cost of capital to make many capital investments. Recently, though, capital costs have been declining, and the company...
-
dvgbbbbhhhcompany that producescleaning products is co nsidering a proposal to begin production of a new detergent that would cost $1 a bottle to make and distribute, and retail for $2.19 a bottle ....
-
You are reviewing audit work papers containing a narrative description of the Tenney Corporations factory payroll sys-tem. A portion of the narrative is as follows: Factory employees punch time clock...
-
How to Interpolate. Apply interpolation to the property data in Table B.2 to determine the following properties of saturated vapor H 2 O:A. The specific volume at 50.8 kPa.B. The saturation...
-
Steam expands is entropically (i.e., at constant entropy s) from 2MPa and 500 K to a final state in which the quality is 0.90. Determine the final-state temperature, pressure, and specific volume. T...
-
Give an example of each of the following, other than those described in this chapter, and clearly explain why your example is this type of relationship and not of some other degree. a. Ternary...
-
What is the length of the partial wavelength for electromagnetic energy with a frequency of 15 MHz and a phase shift of 263 degrees (in meters)?
-
Let y = f(x) be a function that is differentiable at all real numbers. Suppose f has the form f(x) = {; [cx +6 4+2c for x 1 for x > 1. where b and c are some constants. What is the value of the...
-
Consider the sequence (n) defined by xn = (a) Show that 0In - n! nn (b) Use the result of part (a) and the Squeeze theorem to show that In 0 and n o.
-
Officials of Gwinnett County, one of the fastest growing counties in the country, are looking for ways to expand their sewer system. They are considering two alternative sewer designs. All annual...
-
On August 1, 20Y7, Rafael Masey established Planet Realty, which completed the following transactions during the month: Rafael Masey transferred cash from a personal bank account to an account to be...
-
Middleton Corporation just became a public corporation when shares of its stock were sold to the public three months ago. A new board of directors has been appointed to govern the corporation. Assume...
-
Multiple Choice Questions: 1. The largest component of aggregate demand is? a. Government purchases. b. Net exports. c. Consumption. d. Investment. 2. A reduction in personal income taxes, other...
-
During processing in a steel mill, a 750-lb steel casting at 800F is quenched by plunging it into a 500-gal oil bath, which is initially at a temperature of 100F. After the casting cools and the oil...
-
The interior contents and materials of a small building weigh 25 tons, and together they have an average specific heat of 0.25 Btu/(lbm F). Neglecting any inefficiency in the furnace, what amount of...
-
A 5.0-kg steel gear is heated to 150C and then placed into a 0.5-gal container of water at 10C. What is the final temperature of the metal and water?
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


