Consider the equilibrium dissociation of carbon dioxide CO 2 CO + 1/2 O 2 . At
Question:
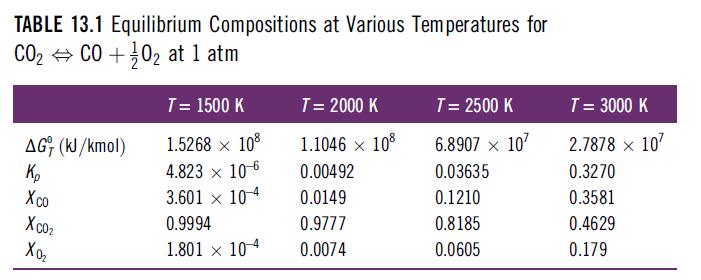
Consider the equilibrium dissociation of carbon dioxide CO2 ⇔ CO + 1/2 O2. At 2500 K, the equilibrium constant is 0.03635. Calculate the enthalpy of reaction for this reaction at 2500 K and use this to estimate the equilibrium constant for a temperature of 3000 K. Compare your estimated value with the exact value from Table 13.1 in Example 13.2 and discuss.
Table 13.1 in Example 13.2
Transcribed Image Text:
TABLE 13.1 Equilibrium Compositions at Various Temperatures for CO₂ CO +02 at 1 atm AGT (kJ/kmol) Кр Xco Xcoz Xo₂ T = 1500 K 1.5268 x 108 4.823 x 10-6 3.601 104 0.9994 1.801 x 104 T = 2000 K 1.1046 × 108 0.00492 0.0149 0.9777 0.0074 T = 2500 K 6.8907 x 107 0.03635 0.1210 0.8185 0.0605 T = 3000 K 2.7878 x 10² 0.3270 0.3581 0.4629 0.179
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To calculate the enthalpy of reaction for the equilibrium dissociation of carbon dioxide at 2500 K w...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The data in the table concern the lactonization of hydroxyvaleric acid at 25oC. They give the concentration C(t)of this acid in moles per liter after minutes. (a) Find the average rate of reaction...
-
Consider the reaction 2 CO2 2 CO + O2 obtained after heating 1 kmol CO2 to 3000 K. Find the equilibrium constant from the shift in Gibbs function and verify its value with the entry in Table A.11....
-
This question draws from the tables in the previous question. Let us try to get an idea of what it would cost an American family at todays prices to purchase the bundle consumed by an average Swedish...
-
Rice and Flower were partners sharing profit and loss equally. Statement of Financial Position as at 31 December 2020. Non current Assets Premises Machinery Vehicles Fittings Current Assets Inventory...
-
Form groups of three to six students each. Each student should pick two companies, preferably from different industries. Find the appropriate data and compute the market-to-book ratio and the ROE for...
-
How does the testing of IT application controls differ from the testing of manual transaction controls and why?
-
Understand the current and long-established fashion calendar. LO.1
-
A recent annual report for FedEx included the following note: Property and Equipment Expenditures for major additions, improvements, flight equipment modifications, and certain equipment overhaul...
-
Problem 22-5A (Part Level Submission) optimus Company manufactures a variety of tools and industrial equipment. The company operates through three divisions. Each division is an investment center...
-
1 Carry out a PESTEL analysis of Alibaba at the time of the case. Evaluate the balance of opportunities and threats, using the same kind of figure as in Illustration 2.1. 2 Draw a basic sociogram of...
-
Ammonia is used as a refrigerant in large-scale refrigeration systems. Using data for ammonia (NH 3 ) from the NIST resources, verify that the condition for phase equilibrium (Eqs.13.35) is met. Use...
-
1. Consolidation workpaper entries normally: a Are posted to the general ledger accounts of one or more of the affiliates b Are posted to the general ledger accounts only when the financial statement...
-
A survey of 214 of the seniors graduating with a bachelor of science degree from a university found that 15% planned to obtain entry-level jobs in the health field. In Exercises 2534, identify the...
-
A retail product has the following standard costs established: Direct Material per unit - 2 pounds at $5 a pound Direct Labor per unit - 3 hours at $12 an hour Manufacturing Overhead - $5 per labor...
-
In a recent year, the Better Business Bureau settled 75% of complaints they received. (Source: USA Today, March 2, 2009) You have been hired by the Bureau to investigate complaints this year...
-
A 1200-ft equal tangent crest vertical curve is currently designed for 50 mph. A civil engineering student contends that 60 mph is safe in a van because of the higher driver's eye height. If all...
-
Required information [The following information applies to the questions displayed below.] Victory Company uses weighted-average process costing to account for its production costs. Conversion cost...
-
Finer, % 100 90 80 70 60 50 40 30 20 10 0 0.01 0.1 1 Size, mm L 10 100 Figure shows a grain size distribution curve of soil. Estimate the coefficient of curvature (Cc) of this soil.
-
In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2 (g). The following data are given at 25°C:
-
Use the following data to answer the next two (2) questions: Product 1 Product 2 Product 3 Direct Material Cost $25,000 $30,000 $35,000 Direct Labor Cost $30,000 $40,000 $50,000 Direct Labor Hours...
-
An engineer is studying the mileage performance characteristics of five types of gasoline additives. In the road test he wishes to use cars as blocks; however, because of a time constraint, he must...
-
Construct a set of orthogonal contrasts for the data in Problem 4-27. Compute the sum of squares for each contrast. Problem 4-27. An engineer is studying the mileage performance characteristics of...
-
Seven different hardwood concentrations are being studied to determine their effect on the strength of the paper produced. However, the pilot plant can only produce three runs each day. As days may...
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...

Study smarter with the SolutionInn App


