Determine the heat pump coefficient of performance for an ideal vapor compression refrigeration cycle operating between 0.653
Question:
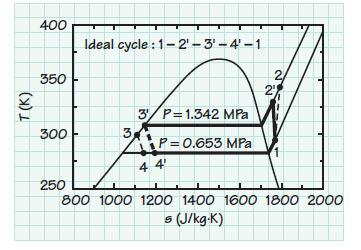
Determine the heat pump coefficient of performance for an ideal vapor compression refrigeration cycle operating between 0.653 and 1.342 MPa. The working fluid is R-22. Compare your result with that for the real cycle described in Example 9.14.
Transcribed Image Text:
Example 9.14 Heat Pump As shown in the diagram below, a heat pump is used to heat a swimming pool by extracting energy from the ambient air at 295.3 K (72 F) and transferring it to the water in the pool at 283.1K (50F). The heat pump operates with R-22 between 0.653 MPa absolute (~80 psig) and 1.342 MPa (~180 psig) and delivers 24.62 kW (84,000 Btu/hr) to the water. The R-22 vapor is superheated to 291.44 K (65F) before it enters the compressor, and the R-22 liquid is subcooled to 299.78 (80F) at the condenser outlet. The scroll compressor has an isentropic efficiency of 65%. Assuming no losses other than those associated with the compressor, plot the vapor-compression cycle in T-s coordinates and determine the cycle coefficient of performance and the mass flow rate of the refrigerant. (Credit: Mario Tama / Staff / Getty Images News.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Determine the influence of the evaporator temperature on the heat pump coefficient of performance for an ideal vapor-compression cycle. The working fluid is R-22. The pressure is fixed in the...
-
An ideal vapor compression refrigeration cycle with R-134a as the working fluid operates between the pressure limits of 120 kPa and 700 kPa. The mass fraction of the refrigerant that is in the liquid...
-
An ideal vapor compression refrigeration cycle with R-134a as the working fluid operates between the pressure limits of 120 kPa and 1000 kPa. The mass fraction of the refrigerant that is in the...
-
Sunland Company has the below information for accruals for the year ended December 31st, 2022. the company adjusts its accounts annually. Chapter 4 Homework e Question 6 of 7 Current Attempt in...
-
Yakima Wheat Company acquired harvesting equipment for $90,000 with an expected useful life of 5 years and a $10,000 expected residual value. Yakima Wheat used straight-line depreciation. During its...
-
Write a description of Tesla's performance over time, including financial and non-financial indicators
-
To an investor, a stock split and a stock dividend have essentially the same effect. Explain the similarity and difference to the corporation between a 100% stock dividend and a 2-for-1 stock split.
-
Omega, Inc., a publicly held corporation, has assets of $100 million and annual earnings in the range of $13 to $15 million. Omega owns three aluminum plants, which are profitable, and one plastics...
-
for risk #2: a. Was the engagement teams assessment of the evaluation of the design of each control appropriate (i.e., does the control identified by the team address the specific risk of material...
-
Distant Ride, Inc. develops and makes a battery for electrical cars. The manufacturing costs per unit include $450 direct materials, $180 direct labor and $300 manufacturing overhead. These costs are...
-
Consider a Rankine cycle steam power plant with superheat having a flow rate of 5 kg/s. The temperatures and pressures at the inlet states of the four basic components are given in the table. The...
-
In a compression-ignition engine, air originally at 50 C is compressed to a temperature of 550 C. The compression obeys the relationship PV 1:34 = constant. Determine (a) The compression ratio...
-
A firm has just developed a new cost-reducing technology for producing a certain replacement part for automobiles. Since a replacement part must be produced within close specifications in order for...
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
A sample of K(s) of mass 2.740 g undergoes combustion in a constant volume calorimeter. The calorimeter constant is 1849 J K 1 , and the measured temperature rise in the inner water bath containing...
-
DEPARTMENT DATA EMPLOYEE DATA EmployeeNumber FirstName Mary Rosalie Richard George Alan 3 4 5 7 8 9 855555ES 12 13 14 15 16 17 Create the database tables in SQL or ACCESS: 18 19 20 PROJECT DATA Ken...
-
Air is compressed by an adiabatic compressor from 100 kPa and 20C to 1.8 MPa and 400C. Air enters the compressor through a 0.15-m 2 opening with a velocity of 30 m/s. It exits through a 0.08-m 2...
-
A 0.5-m 3 rigid tank contains nitrogen gas at 600 kPa and 300 K. Now the gas is compressed isothermally to a volume of 0.2 m 3 . The work done on the gas during this compression process is (a) 82 kJ...
-
Two 10-ft 3 adiabatic tanks are connected by a valve. Initially, one tank contains water at 450 psia with 10 percent quality, while the second contains water at 15 psia with 75 percent quality. The...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


