For the paths indicated in the sketch, show that the entropy change for an ideal gas by
Question:
For the paths indicated in the sketch, show that the entropy change for an ideal gas by either path is the same.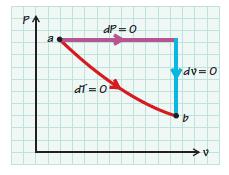
Transcribed Image Text:
PA dP=0 dl=0° dv=0 b A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The entropy change of an ideal gas undergoing a reversible process can be calculated using the equat...View the full answer

Answered By

Joshua Marie Geuvara
I am an academic writer with over 5 years of experience. I write term papers, essays, dissertations, reports, and any other academic paper. My main objective is to produce a high-quality paper free from plagiarism and ensure a student scores an A+. Being a fluent English speaker, I have great communication skills that also enable me to produce excellent papers.
I am conversant with most academic referencing styles (APA, MLA, and Harvard).
You can trust me with your paper and expect nothing less than quality and excellent results. I look forward to meeting with you and, more importantly, developing something that will both make us happy and satisfied.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Using the equation for the entropy change of an ideal gas when the volume and temperature change and TV 1 is a constant, show explicitly that the entropy change is zero for a quasi-static adiabatic...
-
Obtain the relation between the molar entropy change of an ideal gas vapor mixture and the molar entropy of each constituent.
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
If accounting-based performance measures, such as net income or core earnings, are to be significant components of manager compensation plans, they need to be sensitive and precise measures of...
-
Use the information in Exercise to prepare an October statement of retained earnings for Real Answers. In Exercise, On October 1, Keisha King organized Real Answers, a new consulting firm. On October...
-
LO2 Ratliff Development Corporation purchases a tract of land in 2009 at a cost of $120,000 and subdivides the land into 30 building lots. The cost of subdividing is $6,000. In 2009, Ratliff installs...
-
Alcohol-and-marriage study. An experiment was conducted to examine the effects of alcohol on the marital interactions of husbands and wives (Journal of LO4 Abnormal Psychology, Nov. 1998). A total of...
-
Gable Company uses three activity cost pools. Each pool has a cost driver. Information for Gable Company follows: Required: 1. Compute the activity rate for each activity. Round to three decimal...
-
Exercise 18-23 (Algo) Transactions affecting retained earnings (LO18-6, 18-7, 18-8] The balance sheet of Consolidated Paper, Inc., included the following shareholders' equity accounts at December 31,...
-
Draw the free-body diagram for the following problems. a) The beam in Prob. 515. b) The linkage in Prob. 516. c) The frame in Prob. 517. d) The beam in Prob. 518. -8 m- 75 N/m 800 Nm
-
For a simple compressible substance with c p = a(1 + bT), where a and b are constants, determine the entropy change for an isobaric process going from T 1 and T 2 .
-
Air expands through an air turbine from inlet conditions of 690 kPa and 538 C to an exit pressure of 6.9 kPa in an isentropic process. Determine the inlet specific volume, the outlet specific volume,...
-
While above water, a swimmers eyes are focused on a nearby boat. When the swimmer submerges, the underwater part of the boat will not be in focus even though it is the same distance away and the...
-
The combined weight of the load and the platform is 200 lb, with the center of gravity located at G. If a couple moment of M = 900 lb ft is applied to link AB, determine the angular velocity of links...
-
Due In: 06:48:23 Questions Question 1 (4) O Question 2 (8) Question 2 of 2 A company sold $150,000 bonds and set up a sinking fund that was earning 8.5% compounded semi-annually to retire the bonds...
-
Find the point on the graph of f(x) = x which is closest to the point (6, 27). How close is the closest point?
-
Due to a crash at a railroad crossing, an overpass is to be constructed on an existing level highway. the existing highway has a design speed of 50 mi/h. The overpass structure is to be level,...
-
Finding Bone Density Scores. In Exercises 37-40 assume that a randomly selected subject is given a bone density test. Bone density test scores are normally distributed with a mean of 0 and a standard...
-
Consider para-H 2 (B = 60.853 cm -1 ) for which only even J levels are available. Evaluate the rotational partition function for this species at 50. K. Perform this same calculation for HD (B =...
-
As economic conditions change, how do banks adjust their asset portfolio?
-
Members AB and BC can each support a maximum compressive force of 800 lb, and members AD, DC, and BD can support a maximum tensile force of 2000 lb. If a = 6 ft, determine the greatest load P the...
-
Determine the force in each member of the truss and state if the members are in tension or compression. Set P1 = 10 kN, P2 = 8 kN. F E 2 m D. A B 2 m P2
-
Determine the force in each member of the truss and state if the members are in tension or compression. Set P 1 = 8 kN, P 2 = 12 kN. F E 2 m D. A B 2 m P2
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


