For the same conditions as in Problem 4.27, determine the work done if the process is polytropic
Question:
For the same conditions as in Problem 4.27, determine the work done if the process is polytropic with n = 1 (i.e., PV = constant).
Problem 4.27
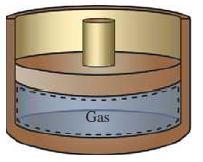
A gas is trapped in a cylinder–piston arrangement as shown in the sketch. The initial pressure and volume are 13,789.5 Pa and 0.02832m3, respectively. Determine the work (kJ) assuming that the volume is increased to 0.08496m3 in a constant-pressure process.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





