In Table C.2, at what reference temperature and pressure is the entropy zero? TABLE C.2 Thermodynamic Properties
Question:
In Table C.2, at what reference temperature and pressure is the entropy zero?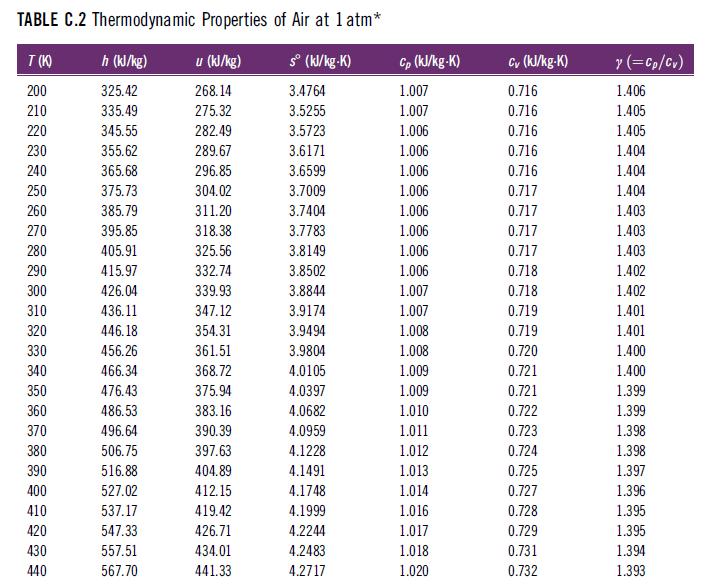
Transcribed Image Text:
TABLE C.2 Thermodynamic Properties of Air at 1 atm* h (kJ/kg) u (kJ/kg) sº (kJ/kg-K) 325.42 268.14 3.4764 335.49 275.32 3.5255 345.55 282.49 3.5723 355.62 289.67 3.6171 365.68 296.85 3.6599 375.73 304.02 3.7009 385.79 311.20 3.7404 395.85 318.38 3.7783 405.91 325.56 3.8149 415.97 332.74 3.8502 426.04 339.93 3.8844 436.11 347.12 3.9174 446.18 354.31 3.9494 456.26 361.51 3.9804 466.34 368.72 4.0105 476.43 375.94 4.0397 486.53 383.16 4.0682 496.64 4.0959 506.75 4.1228 516.88 4.1491 527.02 4.1748 537.17 4.1999 547.33 4.2244 557.51 4.2483 567.70 4.2717 T(K) 200 210 220 230 240 250 260 270 280 290 300 310 320 330 340 350 360 370 380 390 400 410 420 430 440 390.39 397.63 404.89 412.15 419.42 426.71 434.01 441.33 Cp (kJ/kg-K) 1.007 1.007 1.006 1.006 1.006 1.006 1.006 1.006 1.006 1.006 1.007 1.007 1.008 1.008 1.009 1.009 1.010 1.011 1.012 1.013 1.014 1.016 1.017 1.018 1.020 Cv (kJ/kg-K) 0.716 0.716 0.716 0.716 0.716 0.717 0.717 0.717 0.717 0.718 0.718 0.719 0.719 0.720 0.721 0.721 0.722 0.723 0.724 0.725 0.727 0.728 0.729 0.731 0.732 y (=Cp/Cv) 1.406 1.405 1.405 1.404 1.404 1.404 1.403 1.403 1.403 1.402 1.402 1.401 1.401 1.400 1.400 1.399 1.399 1.398 1.398 1.397 1.396 1.395 1.395 1.394 1.393
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Table C2 lists the specific enthalpy h and specific entropy s of various pure substances at differen...View the full answer

Answered By

Joshua Marie Geuvara
I am an academic writer with over 5 years of experience. I write term papers, essays, dissertations, reports, and any other academic paper. My main objective is to produce a high-quality paper free from plagiarism and ensure a student scores an A+. Being a fluent English speaker, I have great communication skills that also enable me to produce excellent papers.
I am conversant with most academic referencing styles (APA, MLA, and Harvard).
You can trust me with your paper and expect nothing less than quality and excellent results. I look forward to meeting with you and, more importantly, developing something that will both make us happy and satisfied.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The following table presents the solubilities of several gases in water at 25 C under a total pressure of gas and water vapor of 1 atm. (a) What volume of CH 4 (g) under standard conditions of...
-
Euler's original article about the Konigsberg Bridge Problem, which is dated 1736, presents a second similar problem with two islands, four rivers flowing around them, and 15 bridges connecting...
-
The molar volume of a certain solid is 142.0 cm-1 mol-1 at 1.00 atm and 427.15 K, its melting temperature. The molar volume of the liquid at this temperature and pressure is 152.6 cm-1 mol-1. At 1.2...
-
Suppose that we want to know the mass of gasoline in an automobile's gas tank. The tank has a volume of 70 L, and a handbook of fluid properties states that the density of gasoline is 736 kg/m. (1000...
-
Reconsider the valuation of the retail clothing chain presented in the previous problem. Use the following benefit stream information for this purpose. You have selected the capitalization of...
-
5. LO.7 During the current year, Gnatcatcher, Inc. (E & P of $1,000,000), distributed $200,000 each to Brandi and Yuen in redemption of some of their Gnatcatcher stock. The two shareholders are not...
-
What are the necessary conditions for use of the Gordon Model?
-
Larissa Company has a unit selling price of $250, variable costs per unit of $170, and fixed costs of $140,000. Compute the break-even point in units using (a) The mathematical equation and (b)...
-
Assume a merchandising companys estimated sales for January and February are $100,000 and $120,000, respectively. Its cost of goods sold is always 40% of its sales. The company always maintains...
-
Hewlett-Packard (HP) is a leading direct marketer of computers and peripherals. The company made a profit of $7,074 million on sales of more than $127 billion in the year ended October 31, 2011. 1....
-
A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and helium mole fractions are 0.12 and 0.35, respectively. If the partial pressure of the nitrogen is 0.8 atm,...
-
A 3-ft 3 rigid vessel contains a 5050 mixture of N 2 and CO (by volume). Determine the mass of each component for T = 65 F and P = 30 psia.
-
(c) How do the results of parts (a) and (b) compare? Explain. (d) Add the probabilities calculated in parts (a) and (b). (e) Are your results in parts (a), (b), and (d) consistent with Fig. 40.5b?...
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Propose a plausible mechanism for the following transformation. Ph H,O* Ph
-
7. FALSE DILEMMA 8. GANDWAGON Definition: Fallacy example: How to revise argument: Definition: Fallacy example: How to revise argument:
-
Determine the mass air-to-fuel ratio (A/F)mass for the combustion of isooctane C 8 H 18 in air. Its stoichiometric equation is C 8 H 18 + 12:5(O 2 + 3:76N 2 ) = 8CO 2 + 9H 2 O + 47:0N 2
-
Assume one kg of isooctane produces 45,500 kJ of energy. How much carbon dioxide is released in obtaining a kJ of energy from the combustion of isooctane in air? (A: 6.79 10 5 kg CO 2 /kJ) Use...
-
Assume a kg of hydrogen in combustion produces 1.20 10 5 kJ of thermal energy. How much carbon dioxide is released in obtaining a kJ of energy from the combustion of hydrogen in air? Use engineering...
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


