Methane stored in a tank at 8 MPa and 300 K is used as a fuel for
Question:
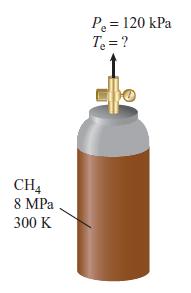
Methane stored in a tank at 8 MPa and 300 K is used as a fuel for a laboratory-scale gas-turbine combustor. The methane is throttled as it passes through a pressure regulator. The regulated pressure is 120 kPa. Determine the temperature of the methane at the exit of the pressure regulator. Assume the process is adiabatic and neglects any kinetic energy changes.
Transcribed Image Text:
CH4 8 MPa 300 K P₂ = 120 kPa T₂ = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To solve this problem we can use the adiabatic throttling equation which rela...View the full answer

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Methane at 10 MPa and 300 K is heated at constant pressure until its volume has increased by 80 percent. Determine the final temperature using the ideal gas equation of state and the compressibility...
-
The vortex tube (also known as a Ranque or Hirsch tube) is a device that produces a refrigeration effect by expanding pressurized gas such as air in a tube (instead of a turbine as in the reversed...
-
Methane gas is stored in a tank at a pressure of 15 bar and 294 K. The tank has a capacity of 0.057 m^3. Gas is allowed to escape from the tank through a partially opened valve. When the pressure in...
-
Assume that everything is the same for the project except the marginal tax rate is now 35%. What is the new NPV? Assume that the cost per unit can be decreased to $12 by using cheaper raw materials...
-
Use the information in the adjusted trial balance reported in Exercise to prepare Resource Trucking Companys classified balance sheet as of December 31, 2011. In Exercise Account Title Debit Credit...
-
Identify someone who owns a timeshare through your family connection, or neighbors and friends. Talk to the person and find out why he/she purchased the timeshare, and identify his/her usage patterns.
-
Is honey a cough remedy? Refer to the Archives of Pediatrics and Adolescent Medicine (Dec. 2007) study of treatments for childrens cough symptoms, Exercise 10.41 (p. 554). Do you agree with the...
-
Reporting Multipurpose Grant Transactions in the Funds and Governmentwide Financial Statements of Local Government Recipients. In this case local governments receive reimbursements from the state...
-
Required information Taylor's is a popular restaurant that offers customers a large dining room and comfortable bar area. Taylor Henry, the owner and manager of the restaurant, has seen the number of...
-
Scott Butterfield is self-employed as a CPA. He uses the cash method of accounting, and his Social Security number is 644-47-7833. His principal business code is 541211. Scott's CPA practice is...
-
Sketch an isentropic and actual pumping process on a Ts diagram for a compressed liquid. Include the vapor dome and the pressure contours that pass through the initial and final states.
-
In a refrigerator, saturated liquid R-134a (a refrigerant) is throttled from an initial temperature of 305 K to a final pressure of 80 kPa. Determine the final temperature and specific volume of the...
-
How could doing a thorough job with Self-Assessment Quiz 1-1 have a major impact on a persons career?
-
The Intel Outsourcing case case explores the make-versus-buy decision for the well-known chip maker. Use The Strategic Sourcing framework to examine this important decision for Intel. Use the...
-
Final-year students enrolled in the Interactive Multimedia course at Edith Cowan University are required to develop skills and expertise in managing the design and development of client websites. The...
-
Find the minimum tractive effort required for vehicle to maintain 70mph speed at 5%upgrade through an air density of 0.002045 slug/ft^3. Show all steps and unit conversion please Problem 2:...
-
Sanburn writes about the conflict of decreasing funding and enrollment for community colleges and the increasing value of an associate degree. Explain how those two factors can co - exist at the same...
-
Rare beauty new Shampoo and Conditioner Branding Strategy What is the branding strategy for your organization? What is the purpose of your brand? How will you differentiate yourself from domestic...
-
In exploring the variation of internal energy with temperature for a two-level system with the ground and excited state separated by an energy of hv, the total energy was found to plateau at a value...
-
A firm has the following balance sheet: Assets Cash Accounts receivable Inventory Plant and equipment $ 15,000 150,000 92,000 170,000 $427,000 Liabilities and Equity Accounts payable Long-term debt...
-
Represent each of the following as a number between 0.1 and 1000 using an appropriate prefix: (a) 45320 kN, (b) 568(105) mm, (c) 0.00563 mg.
-
Round off the following numbers to three significant figures: (a) 58.342 m, (b) 68.534 s, (c) 2553 N, (d) 7555 kg.
-
Represent each of the following combinations of units in the correct SI form using an appropriate prefix: (a) Mg/mm, (b) mN/s, (c) mMg
-
The plant asset and accumulated depreciation accounts of Pell Corporation had the following balances at December 3 1 , 2 0 2 0 : Transactions during 2 0 2 1 were as follows: a . On January 2 , 2 0 2...
-
All else equal, a company's P/E ratio will ____________ when the discount rate ___________. rise, rise fall, rise fall, falls cannot be determined All else equal, a company's P/E ratio will _____...
-
The cost of partially completed goods at the end of the period would be Ending work in process inventory Cost of goods sold Beginning finished goods inventory Beginning work in process inventory

Study smarter with the SolutionInn App


