Use the curve-fit coefficients for the standardized enthalpy from Table H.2 to verify the enthalpies of formation
Question:
Use the curve-fit coefficients for the standardized enthalpy from Table H.2 to verify the enthalpies of formation at 298.15 K in Table H.1 for methane, propane, and hexane.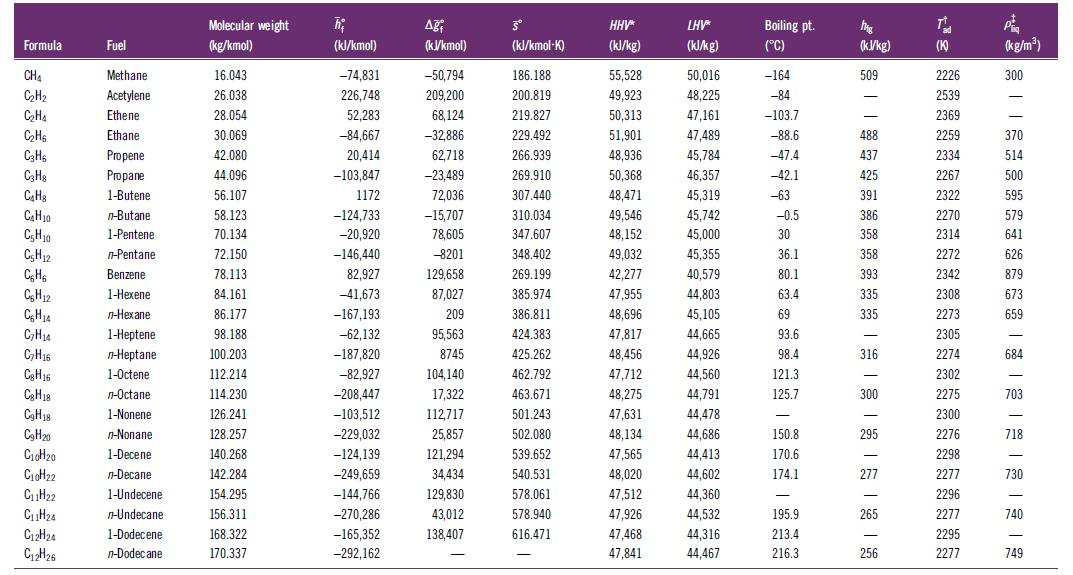
Transcribed Image Text:
Formula CHA C₂H₂ C₂H₁ C₂H6 C3H6 C3H8 C₂H C₂H₂0 C₂H10 C5H12 C₂H6 CH12 C₂H14 C₂H₂4 C₂H16 Cg H16 Cg H18 C₂H18 C₂H20 C₁0H₂0 C₁0H22 C₁1H₂2 C₁1H₂4 C12H24 C12H26 Fuel Methane Acetylene Ethene Ethane Propene Propane 1-Butene n-Butane 1-Pentene n-Pentane Benzene 1-Hexene n-Hexane 1-Heptene n-Heptane 1-Octene n-Octane 1-Nonene n-Nonane 1-Decene n-Decane 1-Undecene n-Undecane 1-Dodecene n-Dodecane Molecular weight (kg/kmol) 16.043 26.038 28.054 30.069 42.080 44.096 56.107 58.123 70.134 72.150 78.113 84.161 86.177 98.188 100.203 112.214 114.230 126.241 128.257 140.268 142.284 154.295 156.311 168.322 170.337 h₂ (kJ/kmol) -74,831 226,748 52,283 -84,667 20,414 -103,847 1172 -124,733 -20,920 -146,440 82,927 -41,673 -167,193 -62,132 -187,820 -82,927 -208,447 -103,512 -229,032 -124,139 -249,659 -144,766 -270,286 -165,352 -292,162 Aği (kJ/kmol) -50,794 209,200 68,124 -32,886 62,718 -23,489 72,036 -15,707 78,605 -8201 129,658 87,027 209 95,563 8745 104,140 17,322 112,717 25,857 121,294 34,434 129,830 43,012 138,407 - Šº (kJ/kmol-K) 186.188 200.819 219.827 229.492 266.939 269.910 307.440 310.034 347.607 348.402 269.199 385.974 386.811 424.383 425.262 462.792 463.671 501.243 502.080 539.652 540.531 578.061 578.940 616.471 - HHV* (kJ/kg) 55,528 49,923 50,313 51,901 48,936 50,368 48,471 49,546 48,152 49,032 42,277 47,955 48,696 47,817 48,456 47,712 48,275 47,631 48,134 47,565 48,020 47,512 47,926 47,468 47,841 LHV* (kJ/kg) 50,016 48,225 47,161 47,489 45,784 46,357 45,319 45,742 45,000 45,355 40,579 44,803 45,105 44,665 44,926 44,560 44,791 44,478 44,686 44,413 44,602 44,360 44,532 44,316 44,467 Boiling pt. (°C) -164 -84 -103.7 -88.6 -47.4 -42.1 -63 -0.5 30 36.1 80.1 63.4 69 93.6 98.4 121.3 125.7 - 150.8 170.6 174.1 - 195.9 213.4 216.3 MAR (kJ/kg) 509 - - 488 437 425 391 386 358 358 393 335 335 316 300 - 295 277 265 256 T (K) 2226 2539 2369 2259 2334 2267 2322 2270 2314 2272 2342 2308 2273 2305 2274 2302 2275 2300 2276 2298 2277 2296 2277 2295 2277 P (kg/m³) 300 370 514 500 595 579 641 626 879 673 659 - 684 - 703 - 718 730 740 749
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (10 reviews)
To verify the enthalpies of formation at 29815 K for methane propane and hex...View the full answer

Answered By

Sigei Kipngeno
I am a professional writer with more than 2 years of writing experience. I will always deliver a grade A paper with zero plagiarism . Thanks in advance.
4.70+
175+ Reviews
311+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
You are required to design an embedded system for a milk production line in a factory. A motor will control a conveyor belt that runs for 4 seconds to position five bottles under five nozzles. The...
-
The standard enthalpy of formation of H2O(l) at 298 K is 285.8 kJ/ mol. Calculate the change in internal energy for the following process at 298 K and 1 atm: H2O(l) H2(g) + O2(g) Eo = ?
-
Have you ever had to cope with the loss of a loved one? If so, what concepts described in this section provide context that may help you understand your experience and process of grieving?
-
Luby Corporation acquires a 100% business use automobile (MACRS 5-year recovery) on July 1, 2010 for $36,000. Luby does not elect Sec. 179, but the company otherwise desires to claim the maximum...
-
A business model analysis provides the auditor with a basis for determining the unique issues that the client faces in its operations by identifying and analyzing the firm's suppliers, competitors,...
-
Why are normalizing adjustments appropriate?
-
The William B. Waugh Corporation is a regional Toyota dealer. The firm sells new and used trucks and is actively involved in the parts business. During the most recent year, the company generated...
-
Cul es el nombre del sistema contable documentado en el siglo XV por Luca Pacioli? A. Gerencial B. Unidad monetaria C. Costo D. Doble entrada
-
Using the property data in Appendix D, reproduce Fig. 13.4. Note that the initial temperature and pressure are 298 K and 1 atm, respectively. Spreadsheet software is recommended to facilitate your...
-
Every Balance Sheet and Statement of Profit and Loss of a company shall comply with: A. US GAAP B. Indian Accounting Standard C. International Accounting Standard.
-
In Exercises 6168, solve each equation. Then determine whether the equation is an identity, a conditional equation, or an inconsistent equation. 4(x + 5) 21+ 4x =
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
A stone is dropped from the roof of a high building. A second stone is dropped 1.30 s later. How far apart are the stones when the second one has reached a speed of 12.0 m / s?
-
What is taxable income, and what is the formula for determining taxable income?
-
Consider a simple ideal Rankine cycle and an ideal regenerative Rankine cycle with one open feedwater heater. The two cycles are very much alike, except the feedwater in the regenerative cycle is...
-
Reconsider Prob. 1034. How much does the thermal efficiency of the cycle change when the temperature at the entrance to the low-pressure turbine is increased to 550C? Data From Q#34: Consider a steam...
-
Consider a steam power plant that operates on the ideal reheat Rankine cycle. The plant maintains the boiler at 17.5 MPa, the reheater at 2 MPa, and the condenser at 50 kPa. The temperature is 550C...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


