What is the temperature or quality of H 2 O in the following states? A:
Question:
What is the temperature or quality of H2O in the following states?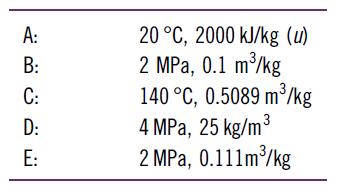
Transcribed Image Text:
A: ننا ت ت ق ة B: C: D: E: 20 °C, 2000 kJ/kg (u) 2 MPa, 0.1 m3/kg 140 °C, 0.5089 m/kg 4 MPa, 25 kg/m 3 2 MPa, 0.111m3/kg
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
ANSWER Using the information provided in the image we can determine the temperature and quality of w...View the full answer

Answered By

Churchil Mino
I have been a tutor for 2 years and have experience working with students of all ages and abilities. I am comfortable working with students one-on-one or in small groups, and am able to adapt my teaching style to meet the needs of each individual. I am patient and supportive, and my goal is to help my students succeed.
I have a strong background in math and science, and have tutored students in these subjects at all levels, from elementary school to college. I have also helped students prepare for standardized tests such as the SAT and ACT. In addition to academic tutoring, I have also worked as a swim coach and a camp counselor, and have experience working with children with special needs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
What is the temperature that corresponds to 1.8-TeV collisions at the Fermilab Collider? To what era in cosmological history does this correspond?
-
What is the temperature inside an ideal refrigerator freezer that operates with a COP = 7.0 in a 22C room?
-
The hottest temperature ever recorded on the surface of the earth was 136F in Libya in 1922. What is the temperature in degrees Celsius and in kelvins?
-
As manager of a local pizza parlor, you want to develop a balanced scorecard so you can more effectively monitor the restaurants performance. Required a. Propose at least two goals for each...
-
Zycor Corporation obtains most of its funding internally. Assume that the stock has a beta of 1.2, the riskless rate is 6.5%, and the market risk premium is 6%. a. Estimate the cost of internal...
-
Joe McGuire is a CPA who has recently completed the audit of Nelson Repairs, Inc. The audited balance sheet and income statement follow. Chapter 4 The Measurement Fundamentals of Financial Accounting...
-
Lack of port facilities or shallow water may require cargo on a large ship to be transferred to a pier using smaller craft. This process may require the smaller craft to cycle back and forth from...
-
Assume that you are assigned the task of testing internal controls over financial reporting for the Van Jacobs Corporation year 2011 audit. Van Jacobs Corporation has a December 31 fiscal year end....
-
If a firm expects cash outflows of equal amounts in two currencies, and the two currencies are ____ correlated, the MNC's transaction exposure is relatively ____.
-
A large manufacturing firm is concerned about lost production (i.e., production capability that was not utilized for a variety of reasons). One of the causes of such lost production was identified as...
-
Steam is condensing in the shell of a heat exchanger at 305 K under steady conditions. The volume of the shell is 2.75m 3 . Determine the mass of the liquid in the shell if the specific enthalpy of...
-
How to Use the NIST Software. Given the following property data for H 2 O, designate the region, line, or point in Tv or Pv space (i.e., compressed liquid, liquidvapor mixture, superheated vapor,...
-
Keep a touch diary for one day of next week and write up your findings for the group. Consider the touching behaviour of your friends with each other: men and men, men and women, women and women,...
-
Sunn Company manufactures a single product that sells for $180 per unit and whose variable costs are $141 per unit. The company's annual fixed costs are $636,000. The sales manager predicts that next...
-
Question 22(5 points) Silver Corp. declares a 15% stock dividend to its shareholders on 1/18. On that date, the company had 15,000 shares issued and 12,000 shares outstanding. Silver Corp. common...
-
Select your a diagnosis from the DSM-5. using your information found through a search of the literature available on your selected diagnosis using appropriate references of peer reviewed journal...
-
Problem 1: Grand Monde Company manufactures various lines of bicycles. Because of the high volume of each type of product, the company employs a process cost system using the FIFO method to determine...
-
Draw the shear force, bending moment diagram of a beam for the loading condition as shown in the figure. Determine the maximum bending moment, and shear force in the beam. Support reactions are pre-...
-
What is the purpose of software testing?
-
Create a data model for one of the processes in the end-of-chapter Exercises for Chapter 4. Explain how you would balance the data model and process model.
-
A natural-gas-fired electrical power plant produces an output of 750 MW. By using a typical efficiency from Table 7.6 and neglecting the small amount of power drawn by the pump, calculate the rates...
-
Suppose that the new type of computer power supply described in Problem P7.31 costs an additional $5. (a) At the cost of 12 per kW ? h, after what period of time would the cost savings in electricity...
-
For the plant in Problem P7.33, 25,000 gal/s of water flow in the river adjacent to the power plant. The river is used as the source of cooling water for the condenser. Considering the heat...
-
Logistics Solutions provides order fulfillment services for dot.com merchants. The company maintains warehouses that stock items carried by its dot.com clients. When a client receives an order from a...
-
Ohno Company specializes in manufacturing a unique model of bicycle helmet. The model is well accepted by consumers, and the company has enough orders to keep the factory production at 10,000 helmets...
-
Entries for Sale of Fixed Asset Equipment acquired on January 5 at a cost of $134,640, has an estimated useful life of 17 years, has an estimated residual value of $9,350, and is depreciated by the...

Study smarter with the SolutionInn App


