Question: A 2 m 3? container for heating water is initially filled with H 2 O liquid and vapor at 400 K, with 20% of the
A 2 m3?container for heating water is initially filled with H2O liquid and vapor at 400 K, with 20% of the volume being liquid. Use the aatables in Appendix A.3 to work this exercise. Check your numbers using the v?u chart, and show the process representation on a v?u sketch.
a. What is the pressure in the tank?b. What is the mass of the H2O in the tank?c. Suppose that the inflow and outflow pipes are closed and the heating occurs at a rate of 170 kW. What will be the temperature in the tank when the pressure has risen to 10 MPa?d. How much energy input as heat will be required to reach this condition?e. How long will it take to reach this condition?
Data From Appendix A.3
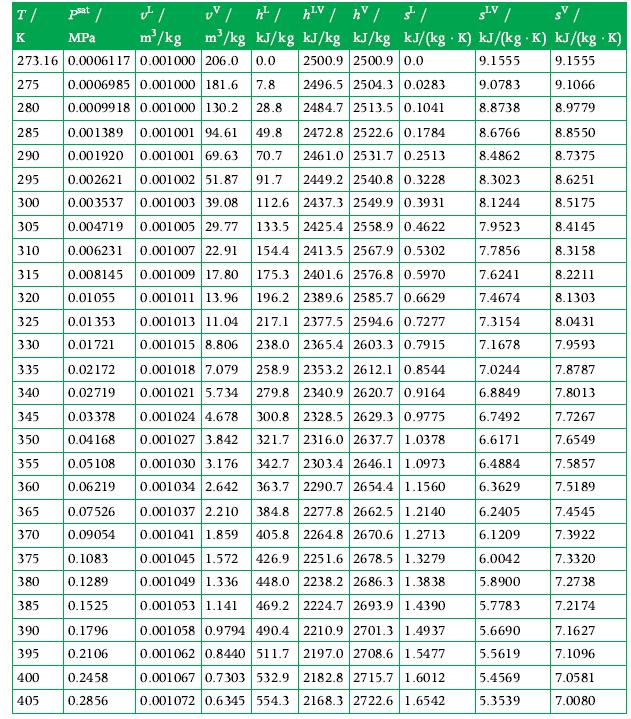
T/ K 275 280 psat / MPa 273.16 0.0006117 0.001000 206.0 0.0 2500.9 2500.9 0.0 2496.5 2504.3 0.0283 2484.7 2513.5 0.1041 285 2472.8 2522.6 0.1784 2461.0 2531.7 0.2513 0.0006985 0.001000 181.6 7.8 0.0009918 0.001000 130.2 28.8 0.001389 0.001001 94.61 49.8 290 0.001920 0.001001 69.63 70.7 295 0.002621 0.001002 51.87 91.7 300 0.003537 0.001003 39.08 112.6 305 0.004719 133.5 2425.4 2558.9 0.4622 310 0.006231 2449.2 2540.8 0.3228 2437.3 2549.9 0.3931 0.001005 29.77 0.001007 22.91 154.4 2413.5 2567.9 0.5302 315 0.008145 175.3 2401.6 2576.8 0.5970 0.001009 17.80 0.001011 13.96 196.2 320 0.01055 2389.6 2585.7 0.6629 217.1 2377.5 2594.6 0.7277 325 0.01 353 330 0.01721 0.001013 11.04 0.001015 8.806 238.0 2365.4 2603.3 0.7915 335 0.02172 340 0.02719 0.03378 0.04 168 345 350 FI UV / h | hV / hv / s/ SLV / m/kg m/kg kJ/kg kJ/kg kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg .K) 9.1555 9.1555 9.0783 9.1066 8.8738 8.9779 0.05108 355 360 0.06219 365 0.07526 370 0.09054 375 0.1083 380 0.1289 385 0.1525 390 0.1796 395 0.2106 400 0.2458 405 0.2856 0.001018 7.079 258.9 2353.2 2612.1 0.8544 0.001021 5.734 279.8 2340.9 2620.7 0.9164 300.8 2328.5 2629.3 0.9775 321.7 2316.0 2637.7 1.0378 342.7 2303.4 2646.1 1.0973 363.7 2290.7 2654.4 1.1560 384.8 405.8 0.001037 2.210 2277.8 2662.5 1.2140 0.001041 1.859 2264.8 2670.6 1.2713 0.001045 1.572 426.9 2251.6 2678.5 1.3279 0.001049 1.336 448.0 2238.2 2686.3 1.3838 0.001053 1.141 469.2 2224.7 2693.9 1.4390 0.001058 0.9794 490.4 2210.9 2701.3 1.4937 0.001062 0.8440 511.7 2197.0 2708.6 1.5477 0.001067 0.7303 532.9 2182.8 2715.7 1.6012 0.001072 0.6345 554.3 2168.3 2722.6 1.6542 0.001024 4.678 0.001027 3.842 0.001030 3.176 0.001034 2.642 8.6766 8.4862 8.3023 8.1244 7.9523 7.7856 7.6241 7.4674 7.3154 7.1678 7.0244 6.8849 6.7492 6.6171 6.4884 6.3629 6.2405 6.1209 6.0042 5.8900 5.7783 5.6690 5.5619 5.4569 5.3539 8.8550 8.7375 8.6251 8.5175 8.4145 8.3158 8.2211 8.1303 8.0431 7.9593 7.8787 7.8013 7.7267 7.6549 7.5857 7.5189 7.4545 7.3922 7.3320 7.2738 7.2174 7.1627 7.1096 7.0581 7.0080
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
Find the saturated properties of water at 400 K from Table A3 Properties of saturated water as a function of temperature V 400k 00010667 mkg V g 400k ... View full answer

Get step-by-step solutions from verified subject matter experts


