An isolated system consists of two rigid subsystems of volumes V 1 and V 2 separated by
Question:
An isolated system consists of two rigid subsystems of volumes V1 and V2 separated by a rigid and porous membrane. Helium (He) can diffuse through the membrane, but oxygen (O2) cannot.We label the gases as A for helium and B for oxygen. The whole system is in thermal equilibrium at all times. Each gas can be considered an ideal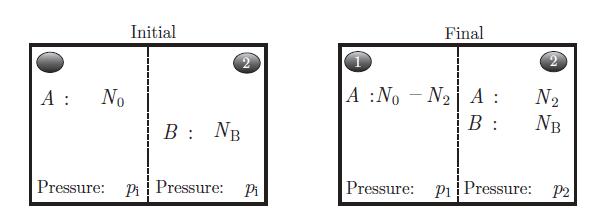 system is divided into two by an osmotic membrane which lets substance A diffuse through it, but not substance B. gas, i.e. they satisfy the equations of state (5.46) and (5.47), namely, pV = NR T and U = cNRT. The gas mixture obeys the ideal mixture relation (8.68), that is,
system is divided into two by an osmotic membrane which lets substance A diffuse through it, but not substance B. gas, i.e. they satisfy the equations of state (5.46) and (5.47), namely, pV = NR T and U = cNRT. The gas mixture obeys the ideal mixture relation (8.68), that is,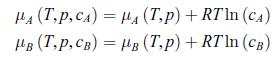 where μA (T, p) and μB (T, p) are the chemical potentials of substances A and B when they are pure, cA and cB are the concentrations of A and B. Initially, the system contains N0 moles of helium in subsystem 1, and NB moles of oxygen in subsystem 2 (Fig. 8.6). The numbers of moles N0 and NB are chosen so that the initial pressure pi is the same in both subsystems. At all times, each subsystem is assumed to be homogeneous. Designate by N1 and N2 the number of moles of helium in subsystems 1 and 2, respectively.a) At equilibrium, show that μA (T, p1) = μA (T, p2, cA).b) Deduce from the previous result a relation between the pressures p1 and p2 when the two sub-systems reach equilibrium. Express cA, p1 and p2 in terms of N2. Determine p1 and p2 in terms of the initial pressure pi under the condition of equal volume, i.e. V1 = V2 = V0.
where μA (T, p) and μB (T, p) are the chemical potentials of substances A and B when they are pure, cA and cB are the concentrations of A and B. Initially, the system contains N0 moles of helium in subsystem 1, and NB moles of oxygen in subsystem 2 (Fig. 8.6). The numbers of moles N0 and NB are chosen so that the initial pressure pi is the same in both subsystems. At all times, each subsystem is assumed to be homogeneous. Designate by N1 and N2 the number of moles of helium in subsystems 1 and 2, respectively.a) At equilibrium, show that μA (T, p1) = μA (T, p2, cA).b) Deduce from the previous result a relation between the pressures p1 and p2 when the two sub-systems reach equilibrium. Express cA, p1 and p2 in terms of N2. Determine p1 and p2 in terms of the initial pressure pi under the condition of equal volume, i.e. V1 = V2 = V0.
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





