Steel wool is placed inside a cylinder filled with molecular oxygen O 2 , considered as an
Question:
Steel wool is placed inside a cylinder filled with molecular oxygen O2, considered as an ideal gas. A piston ensures a constant pressure of the gas. The steel wool reacts with the molecular oxygen to form iron rust Fe2O3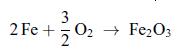 The reaction is slow, so that the gas remains at ambient temperature T0. Determine the heat Qif, the work Wif and the internal energy variation ΔUif in terms of the enthalpy of reaction ΔHif for a reaction involving two moles of iron. Numerical Application:
The reaction is slow, so that the gas remains at ambient temperature T0. Determine the heat Qif, the work Wif and the internal energy variation ΔUif in terms of the enthalpy of reaction ΔHif for a reaction involving two moles of iron. Numerical Application:![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





