Suppose that the silver-silver chloride electrode in Figure 14-2 is replaced by a saturated calomel electrode. Calculate
Question:
Suppose that the silver-silver chloride electrode in Figure 14-2 is replaced by a saturated calomel electrode. Calculate the cell voltage
if [Fe2+] / [Fe3+] = 2.5 × 10-3.
Figure 14-2
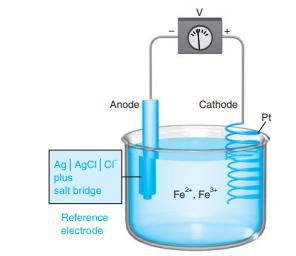
Transcribed Image Text:
Anode Cathode Pt Ag AgCi|cr plus salt bridge Fe", Fe* Reference electrode
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
E E E E 077...View the full answer

Answered By

Payal Mittal
I specialize in finance and accounts.You can ask any question related to til undergradution.Organizational behaviour and HRM are my favourites for you can always relate to them and is an art with practical knowledge base.
4.90+
226+ Reviews
778+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
For a silver-silver chloride electrode, the following potentials are observed: E = 0.222 V E(saturated KCl) = 0.197 V From these potentials, find the activity of Cl - in saturated KCl. Calculate E...
-
A voltaic cell is constructed with two silver-silver chloride electrodes, each of which is based on the following halfreaction: AgCl(s) + e- Ag(s) + Cl- (aq) The two half-cells have [CI-] = 0.0150 M...
-
Suppose that the Ag | AgCl outer electrode in Figure 14-11 is filled with 0.1 M NaCl instead of saturated KCl. Suppose that the electrode is calibrated in a dilute buffer containing 0.1 M KCl at pH...
-
The reconciling item in a bank reconciliation that will result in an adjusting entry by the depositor is: (a) outstanding checks. (b) deposit in transit. (c) a bank error. (d) bank service charges.
-
For the year ended December 31, 2014, Denkinger Electrical Repair Company reports the following summary payroll data. Gross earnings: Administrative salaries ............$200,000 Electricians wages...
-
In a market in which there is vertical differentiation, we always see price differences among the products. In markets with horizontal differentiation, sometimes the products differ but prices are...
-
Learn the CKR Intangible Soft Skills and the CKR Tangible Process Tools to improve your employability and success in the workplace. L01
-
Review each of the following independent sets of conditions. Required: Use AICPA sample size tables to identify the appropriate sample size for use in a statistical sampling application (ROO 5 risk...
-
On June 3, Flounder Company sold to Chester Company merchandise having a sale price of $3,600 with terms of 2/10, n/60, f.o.b. shipping point. An invoice totaling $91, terms n/30, was received by...
-
A ride hailing company has their DB structured in 3 major tables as described in the SCHEMA section below. Write a query to fetch the top 100 users who traveled the most distance using the service....
-
What does the selectivity coefficient tell us? Is it better to have a large or a small selectivity coefficient?
-
Why is it preferable to use a metal ion buffer to achieve pM = 8 rather than just dissolving enough M to give a 10-8 M solution?
-
Congress passed the various pandemic laws without paying for them. The federal deficit is projected to be $2.3 trillion in 2021, equaling 10.3% of GDP. This is the second largest deficit since 1945,...
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
What is a work breakdown structure? What is the difference between a broad-scope work break- down and a narrow-scope work breakdown? Give an example of each.
-
Write the expression in radical notation. Then evaluate the expression when the result is an integer. 23 -1/2
-
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (a) Aqueous acetic acid is neutralized by aqueous barium hydroxide. (b) Solid chromium(III)...
-
Write balanced molecular and net ionic equations for the following reactions, and identify the gas formed in each:
-
Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction...
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


