Estimate E cell for the half reaction. 2H 2 O + 2e - H 2 +
Question:
Estimate E°cell for the half reaction. 2H2O + 2e- → H2 + 2OH- given the following values of ΔGof :
H2O(l) = –237 kJ/ mol H2(g) = 0.0 OH-(aq) = –157 kJ/ mol e- = 0.0 Compare this value of E°cell with the value of E°cell given in Table.
Table
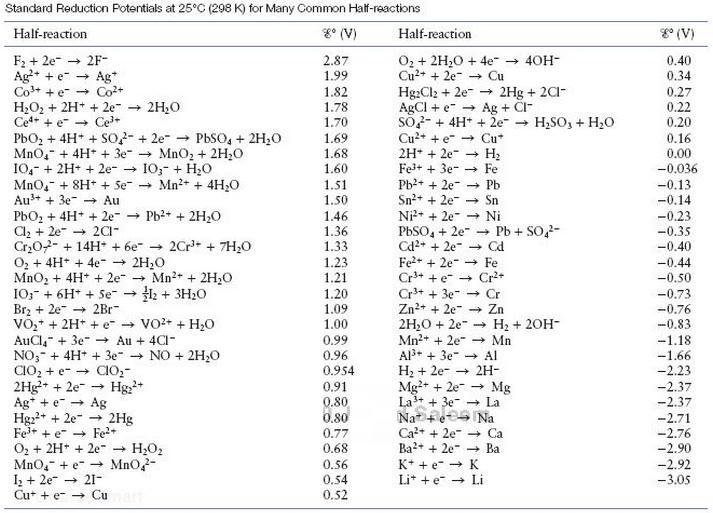
Transcribed Image Text:
Standard Reduction Potentials at 25°C (298 K) for Many Common Half-reactions g° (V) g° (V) Half-reaction Half-reaction O2 + 2H,0 + 4e - 40H- Cu2+ + 2e- - Cu Hg:Cl2 + 2e- AGCI + e- → Ag + Cl- So- + 4H* + 2e- → H,SO, + H20 Cu2+ + e- → Cu* 2H* + 2e Fel+ + 3e- Pb2+ + 2e- Sn2+ + 2e- Ni2* + 2e- → Ni PBSO, + 2e- - Pb + SO2- Cd2+ + 2e- Fe+ + 2e" Cr3+ + e Cr+ Cr+ + 3e- - Cr Zn2+ + 2e Zn 2H;0 + 2e- Mn2+ + 2e" - Mn Al3+ + 3e Al H2 + 2e- Mg+ + 2e → Mg La3+ + 3e- La F2 + 2e- 2F- Ag+ + e Ag* Co+ + e Co+ H2O2 + 2H* + 2e" 2H20 Ce+ + e - Ce+ PbO, + 4H* + SO2- + 2e- - PbSO, + 2H20 Mno,- + 4H* + 3e- → MnO, + 2H;0 10,- + 2H+ + 2e- I0;- + H20 MnO,- + 8H* + Se + Mn2+ + 4H;0 Au3+ + 3e- - Au PbOz + 4H* + 2e- - Pb?+ + 2H20 Cl, + 2e- Cr,02- + 14H + 6e- - O2 + 4H+ + 4e- + 2H,0 MnO, + 4H+ + 2e IO;- + 6H* + Se → H2 + 3H20 Brz + 2e- + 2Br- VO,* + 2H* + e VO2+ + H,0 AuCl, + 3e" - Au + 4CI- NO;- + 4H* + 3e- - NO + 2H20 CIO, + e- → CIO,- 2Hg+ + 2e-- Ag* + e + Ag Hgz2+ + 2e- - 2Hg Fe* + e - Fe+ O, + 2H* + 2e" - H2O2 MnO,- + e- → Mno,- I+ 2e → 21- Cu* + e- → Cu 2.87 0.40 0.34 1.99 - 2Hg + 2C1- 1.82 1.78 1.70 1.69 1.68 1.60 0.27 0.22 0.20 0.16 0.00 -0.036 Н Fe -0.13 -0.14 1.51 1.50 Pb Sn -0.23 1.46 1.36 1.33 1.23 + 201- -0.35 -0.40 2Cr* + 7H,0 - Cd Fe -0.44 Mn2+ + 2H;0 -0.50 1.21 1.20 1.09 1.00 -0.73 -0,76 H2 + 20H- -0.83 0.99 0.96 0.954 -1.18 -1.66 -2.23 + 2H- Hg;+ 0.91 -2.37 -2.37 0.80 0.80 Na Na -2.71 -2.76 -2.90 Ca2+ + 2e- - Ca Ba?+ + 2e" 0.77 0.68 - Ba 0.56 0,54 0.52 K* + e- → K -2.92 Lit +e → Li -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
The two values agree to two significant ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Estimate E cell for the half reaction. 2H 2 O + 2e - H 2 + 2OH - given the following values of G o f : H 2 O(l) = 237 kJ/ mol H 2 (g) = 0.0 OH - (aq) = 157 kJ/ mol e - = 0.0 Compare this value of E...
-
Given the following values of x, s, and n, form a 90% confidence interval for 2. a. x = 21, s = 2.5, n = 50 b. x = 1.3, s = .02, n = 15 c. x = 167, s = 31.6, n = 22 d. x = 9.4, s = 1.5, n = 5
-
Write an equation for the half reaction in which a potassium atom, K, is oxidized.
-
Suppose that you borrow $1000.00 from a friend and promise to pay back $1975.00 in 5 years. What simple interest rate will you pay?
-
Ms. Lynch has a choice of two assets: The first is a risk-free asset that offers a rate of return of rf, and the second is a risky asset (a china shop that caters to large mammals) that has an...
-
Perform instant experiments on whether changing various inputs causes an increase or decrease in the Bond Price and by how much. (a.) What happens when the annual coupon rate is increased? (b.) What...
-
Two-tailed test, n = 14, a = 0.01
-
Alpha Products, Inc., is having a problem trying to control inventory. There is insufi cient time to devote to all its items equally. Here is a sample of some items stocked, along with the annual...
-
If a companys times interest earned ratio goes from 2.7 to 4.9 over a 5 year period, it would indicate that: a) The company is becoming more liquid b) The company is becoming less liquid c) The...
-
Steve, Carol, and Lisa get their first full-time jobs and talk about saving for retirement. They are each 22 years old and plan to work until they are 55. Steve starts investing immediately and puts...
-
When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced equation for the reaction. How many...
-
Glucose is the major fuel for most living cells. The oxidative breakdown of glucose by our body to produce energy is called respiration. The reaction for the complete combustion of glucose is C 6 H...
-
A member of the board of directors is puzzled by the fact that the firm has had a very profitable year but does not have enough cash to pay its bills on time. Explain to the director how a firm can...
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
How do the following matters affect a CPA's reporting responsibilities in performing a compilation? a. A departure from generally accepted accounting principles. b. Inconsistent application of...
-
In Problems 718, write the augmented matrix of the given system of equations. f0.01x0.03y = 0.06 [0.13x + 0.10y = 0.20
-
A political analyst would like to survey a sample of the registered voters in a county. The county has 47 election districts. How could the analyst use random numbers to obtain a cluster sample?
-
Use bond energies (Table 13.6) to show that the pre-ferred products for the decomposition of N2O3 are NO2 and NO rather than O2 and N2O. (The NO single-bond energy is 201 kJ/ mol.) (Hint: Consider...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


