Tell whether each of the following reactions is an oxidation, a reduction, orneither. (a) NABH4 H20 CH;CH-
Question:
Tell whether each of the following reactions is an oxidation, a reduction, orneither.
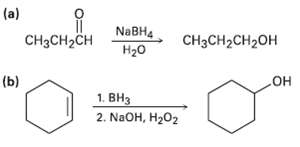
Transcribed Image Text:
(a) NABH4 H20 CH;CH-сн CH3CH2CH2OH (b) OH 1. BH3 2. NaOH, H202
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a The aldehyde carbon of the reactant has an oxidation level o...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Indicate whether each of the following reactions is an oxidation reaction, a reduction reaction, or neither: a. b. c. d. e. f. H2 CHj 3CI partially deactivatedCH Pd HBr RCH CHRRCH2CHR Br2 H2CrO4...
-
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, orE2: NaN3 (a) CHCH2CH2H2Br CH3CH2CH2CH,N=N=N THE CI (b) , CCH-CHCH2CH CHCH2CHCHCH3 Ethanol (c) H CI -CH -- -CH (d)...
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
United Research Associates (URA) had received a contract to produce two units of a new cruise missile guidance control. The first unit took 4,000 hours to complete and cost $ 30,000 in materials and...
-
Brad's Burritos provides a simple set of lunch offerings in a college town. The shop has recently decided to implement a forecasting system and is testing the use of exponential smoothing methods....
-
Liquid nitrogen at 77 K is stored in a cylindrical container having an inside diameter of 25 cm. The cylinder is made of stainless steel and has a wall thickness of 1.2 cm. Insulation is to be added...
-
On April 29, Kirsten Fletcher and John E. Marshall III jointly signed a lease to rent an apartment for the term beginning on July 1 and ending on June 30 of the following year, for a monthly rent of...
-
On March 11, 20XX, the existing or current (spot) 1-, 2-, 3-, and 4-year zero-coupon Treasury security rates were as follows: 1R1 = 0.75%, 1R2 = 1.35%, 1R3 = 1.75%, 1R4 = 1.90% Using the unbiased...
-
.Determine whether the following statements are TRUE or FALSE. Briefly explain your answer. (a) A cryptocurrency, e.g. Bitcoin, is a productive asset. (b) The rate of returns of a bond is always the...
-
Impala Window Washing Services prepares adjustments monthly and shows the following selected accounts on its December 31, 2014, unadjusted trial balance: Required Prepare the required monthly...
-
Rank each of the following series of compounds in order of increasing oxidationlevel: CI (a) (b) CH3CN CH3CH2NH2 H2NCH2CH2NH2
-
Give a JUPAC name for each of the following alkyl halides (yellow green =Cl): (b) (a)
-
Describe your personality by listing 710 adjectives that illustrate your behavior and approach to life. Now imagine your opposite personality type by listing 710 adjectives that are diametrically...
-
February 12, 2009 marked the 200th anniversary of Charles Darwin's birth. To celebrate, Gallup, a national polling organization, surveyed 1,018 randomly selected American adults about their education...
-
Question 1 (30 points) A 3D infinite quantum well is a very simple model for an atom. Suppose that two cubic 3D infinite quantum wells, with cube dimension L, are joined to form one parallelepiped...
-
Give an algorithm for printing all the ancestors of a node in a Binary tree. For the tree below, for 7 the ancestors are 137. root 4 2 3 5 6 7
-
Consider k measurements that are corrupted by zero-mean Gaussian noise with s.d. , i.e., z=x+wi, i = 1,..., k (6) where x is a constant and w; ~N(0,0). The goal is to estimate the mean x and the...
-
1. For an inviscid flow, the momentum equation for a Newtonian flow can be written as: -(puu;)+ Jxi (pu)+ at 2x j where p is the density and is the pressure. = = 0 (1) (a) In order to characterise a...
-
Most new-employee orientation sessions in small hospitality operations take less than two hours to complete. A. True B. False
-
Maria Castigliani is head of the purchasing department of Ambrosiana Merceti, a medium-sized construction company. One morning she walked into the office and said, The main problem in this office is...
-
Which statement best describes the difference between the charge of a polyatomic ion and the oxidation states of its constituent atoms? (For example, the charge of NO 3 - is 1, and the oxidation...
-
Draw the structure that corresponds with each name. (a) 3-ethyloctane (b) 4-isopropyldecane (c) Sec-butylcycloheptane (d) 2,3-dimethyl-4-propylnonane (e) 2,2,4,4-tetramethylhexane (f)...
-
Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description. (a) An isopropylheptane (b) A diethyldecane (c) A...
-
Give the IUPAC names of the following alkanes. (a) CH3C(CH3)2CH(CH)CH3)CH2CH2CH(CH3)2 (b) (c) (d) (e) (f) (g) (h) CH,CH CHCH CH, CH CH CH,CHCH CH,CHCH CH,CH CH,CH, CH, CH,CH, CH,CH,CH, C(CH,CH),...
-
You plan to buy a house for $325,000 today. If the house is expected to appreciate in value 8% each year, what will its value be seven years from now?
-
A designated beneficiary of an ABLE account must be ___________ in order to meet the special rules that apply to the increased contribution limit authorized under the Tax Cuts and Jobs Act? a. an...
-
Stans wholesale buys canned tomatoes from canneries and sells them to retail markets Stan uses the perpetual inventory

Study smarter with the SolutionInn App


