The capacitors in the circuit in Figure are initially uncharged. (a) What is the initial value of
Question:
The capacitors in the circuit in Figure are initially uncharged.
(a) What is the initial value of the battery current when switch S is closed?
(b) What is the battery current after a long time?
(c) What are the final charges on thecapacitors?
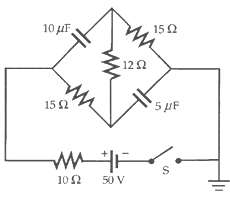
Transcribed Image Text:
10 uF 150 12 2 15 2 5 pF 10 2 50 V %24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
a Since V C 0 for both capacitors the resistors are effectively in parall...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Electricity and Magnetism questions
-
The initial conditions in the circuit in figure are zero. Find the transfer function Io(s) /Is(s). is(t) s(s + 4) a. s + 4s + 3 b. s + 3s + 1 C. s? + 5s + 7 d. s2 + 2s + 10 w-
-
The capacitors in each circuit are discharged when the switch closes at t = 0 s. Rank in order, from largest to smallest, the time constants t1 to t5 with which each circuit will discharge. www ww
-
Find the voltage across the capacitors in the circuit of Fig. 6.49 under dc conditions. 10 50 20 (2 30 60 Y
-
What is the difference between a Type I error and a Type II error?
-
Janet, Brian, and Natalie have decided to take on the additional work offered by Coffee Beans. Recall that Coffee Beans is doubling the number of cupcakes required on a weekly basis. The Koebels are...
-
Light is incident on a diffraction grating at angle a to the normal. Show that the condition for maximum light intensity becomes d(sinsin) = m.
-
True or false. H0: s1 2 = s2 2 is equivalent to H0: s1 2 >s2 2 LO3 = c where c 1.
-
South Atlantic Chemical Company manufactures industrial chemicals in Rio de Janeiro, Brazil. The company plans to introduce a new chemical solution and needs to develop a standard product cost. The...
-
Cabanos Company manufactures two products, Product C and Product D. The company estimated it would incur a total of $160,790 in manufacturing overhead costs during the current period. Overhead...
-
The T-accounts below summarize the ledger of Santana Landscaping Company at the end of its first month of operations. Instructions (a) Prepare the complete general journal (including explanations)...
-
A closed box has two metal terminals a and b. The inside of the box contains an unknown emf E in series with a resistance R. When a potential difference of 21 V is maintained between a and b, there...
-
The circuit in Figure is a slide-type Wheatstone bridge.?It is used for determining an unknown resistance R x in terms of the known resistances R 1 , R 2 , and R 0 . The resistances R 1 and R 2...
-
The 1H NMR data for the two anomers included very comparable peaks in the d 2.0-5.6 region but differed in that, as their highest peaks, anomer V had a doublet at d 5.8 (1H, J = 12 Hz) while anomer...
-
8 Project two 15 UTSA Project two M Question 1 - Project two ChatGPT C chegg.com/homework-he X Course Hero how to take a sxreen shot X +...
-
Charitable purposes: Section 3(1) Charities Act 2011 1. Prevention or relief of poverty 2. Education 3. Religion, now includes: 4. - - A religion which involves belief in more than one god A religion...
-
Jack Price, The finance director of Humpty Doo Investment Ltd ( HDIL ) , is unsure whether he should consolidate some of the investments that the company owns. He has asked your advice as business...
-
Use QM to solve this problem. Suppose that Peter Cartman is deciding whether to invest in a bond mutual fund or a stock fund. Both bond and stock funds are sensitive to changing market conditions....
-
George Francis works at Gentry Medical Center which is in sunny Florida. The Medical Center experiences a higher volume of business closer to fall when many of the patients return for the winter from...
-
What actions, if any, could Sony employees take to protect themselves from the theft and release of their data on their employers servers?
-
Find the APR in each of the following cases: NUMBER OF TIMES COMPOUNDED Semiannually Monthly Weekly Infinite EAR APR 10.4% 8.9 11.6 15.4
-
Examine potentially effective trading strategies for CEFs.
-
A vessel contains a mixture of nitrogen (m1 = 7.0 g) and carbon dioxide (m2 = 11 g) at a temperature T = 290 K and pres- sure P0 = 1.0 atm. Find the density of this mixture, assuming the gases to be...
-
A vessel of volume V = 7.5 1 contains a mixture of ideal gases at a temperature T = 300 K: v1 = 0.10 mole of oxygen, v2 = 0.20 mole of nitrogen, and v3 = 0.30 mole of carbon dioxide. Assuming the...
-
A vertical cylinder closed from both ends is equipped with an easily moving piston dividing the volume into two parts, each containing one mole of air. In equilibrium at To = 300 K the volume of the...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


