The distribution coefficient for extraction of a metal complex from aqueous to organic solvents is D =
Question:
The distribution coefficient for extraction of a metal complex from aqueous to organic solvents is D = [total metal]org/[total metal]aq. Give physical reasons why β and Ka appear in the numerator of Equation 22-13, but KL and [H+]aq appear in the denominator.
Equation 22-13
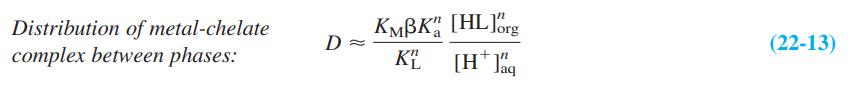
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Transcribed Image Text:
KMBK", [HL]brg Distribution of metal-chelate complex between phases: (22-13) KE [H* J%q
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
The form that is extracted into organic solvent is MLW The formation of ML n is f...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
For the extraction of Cu 2+ by dithizone in CCl 4 , KL= 1.1 10 4 , K M = 7 10 4 , K a = 3 10 - 5 , = 5 10 22 , and n = 2. (a) Calculate the distribution coefficient for extraction of 0.1 M Cu 2+...
-
An aqueous acetic-acid solution containing 6.0 moles of acid per liter is to be extracted in the laboratory with chloroform at 25oC to recover the acid (B) from chloroform-insoluble impurities...
-
Consider the extraction of 100.0 mL of M2+ (aq) by 2.0 mL of 1 10-5 M dithizone in CHCl3, for which KL = 1.1 104, KM = 7 104, Ka = 3 10-5, = 5 1018, and n = 2. (a) Derive an expression for the...
-
Analyze how you will use the challenge the process and enable others to act practices to improve the three leadership areas that you selected in module one. ? select one leadership theory or approach...
-
At May 31, Suarez Company has net sales of $330,000 and cost of goods available for sale of $230,000. Compute the estimated cost of the ending inventory , assuming the gross profit rate is 35%.
-
The tort of defamation does not occur unless a defamatory statement is made in writing. (True/False)
-
5-8. Qu tipos de grupos de referencia interesan a los mercadlogos?
-
Calculating OCF Ranney, Inc., has sales of $18,700, costs of $10,300, depreciation expense of $1,900, and interest expense of $1,250. If the tax rate is 40 percent, what is the operating cash flow,...
-
Required information (The following information applies to the questions displayed below.) Kubin Company's relevant range of production is 29,000 to 33,000 units. When it produces and sells 31,000...
-
Using the information from the following table, create an AON network activity diagram. a. Calculate each activity TE (rounding to the nearest integer); the total duration of the project; its early...
-
Consider two Gaussian peaks with relative areas of 4:1. Construct a set of graphs to show the overlapping peaks if the resolution is 0.5, 1, or 2.
-
Give a physical interpretation of Equations 22-6 and 22-7 in terms of the fractional composition equations for a monoprotic acid discussed in Section 9-5
-
Taking the value of xx at 0 to be 1 and integrating a series term by term, show that 1n
-
Please help! I'm stuck 1) What purpose would your computer system serve? Business or personal or both? 2) Is this laptop/portable or desktop with monitor attached or all-in-one desktop? 3) What would...
-
The airline industry is severely hit by the COVID-19. Rows 6 to 85 show the daily closing prices of three stocks (i.e.,Qantas Airways Limited (QAN.AX), Singapore Airlines Limited (C6L.SI), and Cathay...
-
Using C+ Write a program to let users input two integers. If the first number is greater than the second number, print "The first number is larger". If the second number is greater than the first...
-
7. The normal model Show that if the risk-neutral distribution of ST is given by ST | S ~N (F, (T-t)), where F = F(t, T)istheforwardprice, thenthepriceofa K-strike straddle is approxim- ated by Z(t,...
-
25 cm 75 cm Water Parabola 2. The wheel-well of a custom truck-mounted water tank has a semi- parabolic shape as shown (assume point A corresponds to the peak). It's width is projected 150 cm into...
-
Identify the set of hybrid orbitals of a central atom that forms bonds with the following angles. (a) 120 degrees (b) 90 degrees (c) 180 degrees
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
Bone consists of the protein collagen and the mineral hydroxyapatite, Ca 10 (PO 4 ) 6 (OH) 2 . The Pb content of archaeological human skeletons measured by graphite furnace atomic absorption sheds...
-
Why is an internal standard most appropriate for quantitative analysis when unavoidable sample losses are expected during sample preparation?
-
Explain why X-ray fluorescence is observed when matter absorbs X-rays of sufficient energy. Why does each element have a unique X-ray signature?
-
Hrubec Products, Incorporated, operates a Pulp Division that manufactures wood pulp for use in the production of various paper goods. Revenue and costs associated with a ton of pulp follow: Selling...
-
The AICPA guidelines suggest that taxes should be transparent and visible. This means that: a. The taxes affect similarly situated taxpayers in a similar manner. b. Taxes should be due at the same...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...

Study smarter with the SolutionInn App


