The following data were obtained when a Ca 2+ ion-selective electrode was immersed in standard solutions whose
Question:
The following data were obtained when a Ca2+ ion-selective electrode was immersed in standard solutions whose ionic strength was constant at 2.0 M.
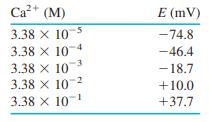
(a) Prepare a calibration curve and find the least-squares slope and intercept and their standard deviations.
(b) Calculate the value of in Equation 14-13.
(c) For a measured potential, the calibration curve gives us log[Ca2+]. We can compute [Ca2+] = 10log[Ca2+] . Using rules for propagation of uncertainty in Table 3-1, calculate [Ca2+] and its associated uncertainty of a sample that gave a reading of -22.5 (± 0.3) mV in four replicate measurements.
Equation 14-13

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





