The heat capacity at constant volume of hydrogen sulfide at low pressures is C v [kJ/ (mol??C)]
Question:
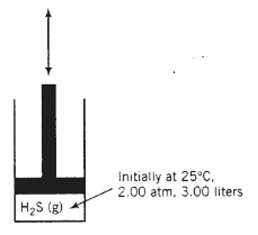
The heat capacity at constant volume of hydrogen sulfide at low pressures is Cv [kJ/ (mol??C)] = 0.0252 + 1.547 x 10?5?T ? 3.012 X 10?9T2?where T is in ?C. A quantity of H2S is kept in a piston-fitted cylinder with initial temperature, pressure, and volume equal to 25?C, 2.00 atm, and 3.00 liters, respectively.
(a) Calculate the heat (kJ) required to raise the gas temperature from 25?C to 1000?C if the heating takes place at constant volume (i.e., if the piston does not move), retaining successively one term, two terms, and all three terms of the heat capacity formula. (See Example 8.3-1) Determine the percentage errors in Q that result from retaining only one and two terms of the heat capacity formula, assuming that the full expression yields the correct result.
(b) For a closed system at constant pressure with negligible kinetic and potential energy changes, the energy balance equation is Q = ?H. Use Equation 8.3-12 to determine an expression for the heat capacity at constant pressure (Cp) for H2S, assuming ideal gas behavior. Then use it to calculate the heat (J) required to raise the gas from 2.5?C to 1000?C at constant pressure. What would the piston do during this process?
(c) What is the physical significance of the difference between the values of Q calculated in parts (a) and (b)?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





