The metal ion indicator xylenol orange (Table 11-3) is yellow at pH 6 ( max = 439
Question:
The metal ion indicator xylenol orange (Table 11-3) is yellow at pH 6 (λmax = 439 nm). The spectral changes that occur as VO2+ is added to the indicator at pH 6 are shown here. The mole ratio VO2+/xylenol orange at each point is
-1.png)
Suggest a sequence of chemical reactions to explain the spectral changes, especially the isosbestic points at 457 and 528 nm.
-2.png)
Table 11-3
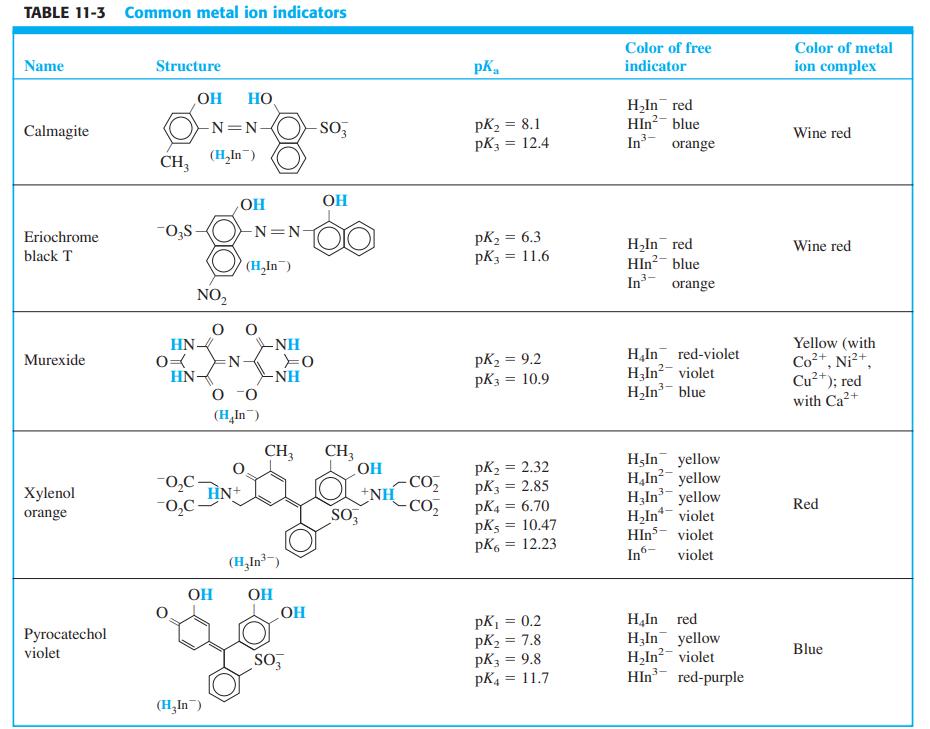
Transcribed Image Text:
Trace Mole ratiTrace Mole ratio Trace Mole ratio 0.60 0.70 0.80 0.90 1.0 12 13 1.5 14 2.0 15 3.1 16 4.1 1.3 0.10 2 0.20 0.30 0.40 5 0.50 4 10 16 0.1 Absorbance unit 13 12 600 500 Wavelength (nm) 400
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
As VO 2 is added traces 19 the peak at 439 decreases a...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A 1.000-mL sample of unknown containing Co2+ and Ni2+ was treated with 25.00 mL of 0.03872 M EDTA. Back titration with 0.02127 M Zn2+ at pH 5 required 23.54 mL to reach the xylenol orange end point....
-
A novel metal ion adsorbent material based on the bio polymer poly glucosamine is cast into a gel bead. The amine groups on the bio polymer have a high affinity for transition metal ions at...
-
The amount of lactic acid, HC3H5O3, produced in a sample of muscle tissue was analyzed by reaction with hydroxide ion. Hydroxide ion was produced in the sample mixture by electrolysis. The cathode...
-
Does the fact that the strategic petroleum reserve has never been used to offset shortfalls caused by an embargo mean that the money spent in creating the reserve has been wasted? Why or why not?
-
Farwell Company closes its books monthly. On September 30, selected ledger account balances are: Notes Receivable .......$37,000 Interest Receivable ....... 183 Notes Receivable include the...
-
REI sells snowboards. Assume the following information relates to REI's purchases of snowboards during September. During the same month, 102 snowboards were sold. REI uses a periodic inventory...
-
9 Describa las estrategias de administracin de la capacidad que una aerolnea debe tomar en consideracin.
-
The following equation summarizes the trend portion of quarterly sales of condominiums over a long cycle. Sales also exhibit seasonal variations. Using the information given, prepare a forecast of...
-
Taser Company has to purchase some new equipment. Two manufacturers have provided the following information: Equipment A Equipment B $90,000 $67,500 Initial costs Estimated life Annual savings 5...
-
On July 1, 2017, Major Co. pays $15,120 to Cruz Insurance Co. for a 3-year insurance contract. Both companies have fiscal years ending December 31. For Major Co., journalize and post the entry on...
-
When are isosbestic points observed and why?
-
Infrared spectra are customarily recorded on a transmittance scale so that weak and strong bands can be displayed on the same scale. The region near 2 000 cm -1 in the infrared spectra of compounds A...
-
Which of the following is a security? I. Bond. II. LLC membership interest. III. Mortgage. IV. Stock. A. I, IV. B. I, III, IV. C. IV. D. I, II, III, IV.
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
What is the purpose of crashing the schedule?
-
Consider the setup in Problem 16. Show that the relative speed of the ball and the point of contact on the stick is the same before and immediately after the collision. (This result is analogous to...
-
Select indicators from Table 10-3 that would be useful for the titrations in Figures 10-1 and 10-2 and the pK a = 8 curve in Figure 10-3. Select a different indicator for each titration and state...
-
When 100.0 mL of a weak acid were titrated with 0.093 81 M NaOH, 27.63 mL were required to reach the equivalence point. The pH at the equivalence point was 10.99. What was the pH when only 19.47 mL...
-
A 0.100 M solution of the weak acid HA was titrated with 0.100 M NaOH. The pH measured when V b = V e was 4.62. Using activity coefficients, calculate pKa. The size of the A - anion is 450 pm.
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


