Question: The p-V diagram in Figure shows two paths along which a sample of gas can be taken from state a to state 4 where Vb
The p-V diagram in Figure shows two paths along which a sample of gas can be taken from state a to state 4 where Vb = 3.0V1. Path 1 requires that energy equal to 5.0p1V1 be transferred to the gas as heat. Path2 requires that energy equal to 5.5p1V1 be transferred to the gas as heat. What is the ratiop2/p1.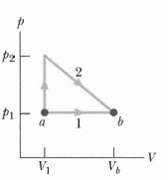
A Vs
Step by Step Solution
3.33 Rating (156 Votes )
There are 3 Steps involved in it
Since the combination pV appears frequently in this derivatio... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-F-L (489).docx
120 KBs Word File


