Question: The sharp separation of benzene and cyclohexane by distillation at ambient pressure is impossible because of the formation of an azeotrope at 77.6oC. K.C. Chao
The sharp separation of benzene and cyclohexane by distillation at ambient pressure is impossible because of the formation of an azeotrope at 77.6oC. K.C. Chao [Ph.D. thesis, University of Wisconsin (1956)] obtained the following vapor-liquid equilibrium data for the benzene (B)/cyclohexane (CH) system at 1 atm: Vapor pressure is given by (2-39), where constants for benzene are in Exercise 2.12 and constants for cyclohexane are kl = 15.7527, k2 = -2766.63, and k3 = -50.50.(a) Use the data to calculate and plot the relative volatility of benzene with respect to cyclohexane versus benzene composition in the liquid phase. What happens to the relative volatility in the vicinity of the azeotrope?(b) From the azeotropic composition for the benzeneicyclohexane system, calculate the constants in the van Laar equation. With these constants, use the van Laar equation to compute the activity coefficients over the entire range of composition and compare them, in a plot like Figure, with the above experimental data. How well does the van Laar equation predict the activity coefficients?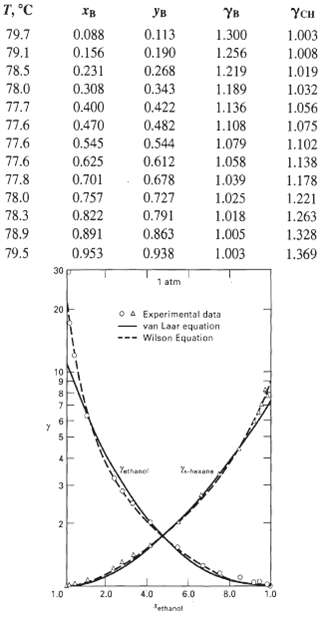
, YB 0.113 79.7 0.088 1.300 1.003 79.1 0.156 0.190 1.256 1.008 0.231 78.5 0.268 1.219 1.189 1.136 1.019 78.0 0.308 0.343 1.032 77.7 0.400 0.422 1.056 77.6 0.470 0.482 1.108 1.075 77.6 0.545 0.544 1.079 1.102 77.6 0.625 0.612 1.058 1.039 1.138 0.701 77.8 0.678 1.178 78.0 0.757 0.727 1.025 1.221 0.791 78.3 0.822 1.018 1.263 78.9 0.891 0.863 1.328 1.005 79.5 0.953 0.938 1.003 1.369 30 1 atm 20 O A Experimental data van Laar equation Wilson Equation Tethane 2.0 4.0 6.0 B.0 1.0 1.0 Tethanat
Step by Step Solution
3.27 Rating (168 Votes )
There are 3 Steps involved in it
a From Eqs 221 219 and 3 in Table 23 Using the yx data for benzene ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (47).docx
120 KBs Word File


