Question: The strong neutron excess (defined as N - Z) of high-mass nuclei is illustrated by noting that most high-mass nuclides could never fission into two
The strong neutron excess (defined as N - Z) of high-mass nuclei is illustrated by noting that most high-mass nuclides could never fission into two stable nuclei without neutrons being left over. For example, consider the spontaneous fission of a 235U nucleus into two stable daughter nuclei with atomic numbers 39 and 53. From Appendix F, determine the name of the
(a) First and
(b) Second daughter nucleus. From Figure, approximately how many neutrons are in the (c) First and
(d) Second?
(e) Approximately how many neutrons are left over?
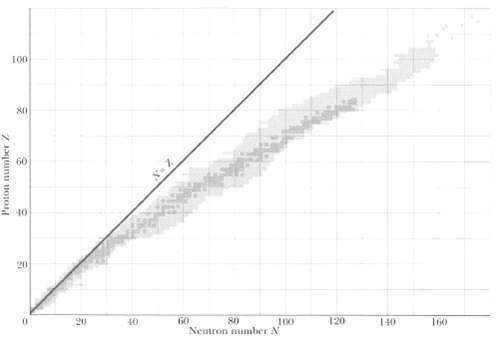
100 60 40 20 60 80 Neutron number N 120 20 40 100 140 160 Proton number /
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
a The atomic number Z 39 corresponds to the element yttrium see Appendix F andor Appendix G b T... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-M-P-N-P (191).docx
120 KBs Word File


