Question: The unit cell for MgFe2O4 (MgO-Fe2O3) has cubic symmetry with a unit cell edge length of 0.836 nm. If the density of this material is
The unit cell for MgFe2O4 (MgO-Fe2O3) has cubic symmetry with a unit cell edge length of 0.836 nm. If the density of this material is 4.52 g/cm3, compute its atomic packing factor. For this computation, you will need to use ionic radii listed in Table12.3.
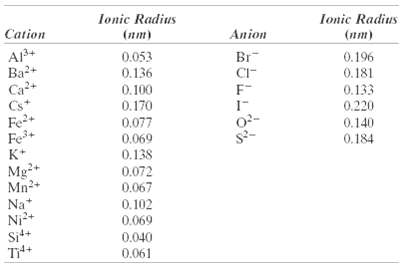
Ionic Radius Ionic Radius Cation (nm) Anion (nm) Aj3+ Ba2+ Ca+ Cs* 0.053 Br 0.196 0.136 CI- F- 0.181 0.100 0.170 0.133 0.220 Fe2+ Fe+ K+ 0.077 0.069 0.138 0.072 0.067 0.140 0.184 Mg-+ Mn2+ Na* Ni+ Sit+ 0.102 0.069 0.040 0.061
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
This problem asks us to compute the atomic packing factor for MgFe 2 O 4 giv... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (479).docx
120 KBs Word File


