Treatment of 1, 1-diphenyl- 1, 2-epoxyethane with aqueous acid yields diphenylacetaldehyde as the major product. Propose a mechanism for the reaction. Ph H30+ PHCHCH Ph
Treatment of 1, 1-diphenyl- 1, 2-epoxyethane with aqueous acid yields diphenylacetaldehyde as the major product. Propose a mechanism for the reaction.
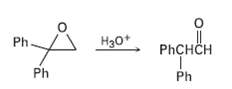
Ph H30+ PHCHCH Ph Ph
Step by Step Solution
3.34 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
HOH PhCCH PhCCH OH PhCCH HO PhCHCH H h... View full answer

Get step-by-step solutions from verified subject matter experts
100% Satisfaction Guaranteed-or Get a Refund!
Step: 2Unlock detailed examples and clear explanations to master concepts

Step: 3Unlock to practice, ask and learn with real-world examples

Document Format ( 1 attachment)
22-C-O-E (87).docx
120 KBs Word File
See step-by-step solutions with expert insights and AI powered tools for academic success
-
 Access 30 Million+ textbook solutions.
Access 30 Million+ textbook solutions.
-
 Ask unlimited questions from AI Tutors.
Ask unlimited questions from AI Tutors.
-
 Order free textbooks.
Order free textbooks.
-
 100% Satisfaction Guaranteed-or Get a Refund!
100% Satisfaction Guaranteed-or Get a Refund!
Claim Your Hoodie Now!

Study Smart with AI Flashcards
Access a vast library of flashcards, create your own, and experience a game-changing transformation in how you learn and retain knowledge
Explore Flashcards





