Turpentine, obtained from pine trees, is composed primarily of ?-pinene and ?-pinene, Explain whether you expect turpentine
Question:
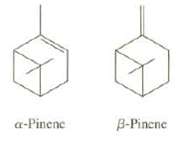
Turpentine, obtained from pine trees, is composed primarily of ?-pinene and ?-pinene, Explain whether you expect turpentine to mix with water. If a point dissolves in turpentine, what does this suggest about the structure of the paint?
Transcribed Image Text:
a-Pinene B-Pinene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
Turpentine should not mix with wa...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
If streptomycin blocks protein synthesis in organelles, what does this tell you about the organelles relationship to Bacteria?
-
Gasoline is composed primarily of hydrocarbons, including many with eight carbon atoms, called octanes. One of the cleanest-burning octanes is a compound called 2,3,4- trimethylpentane, which has the...
-
The diameters (Y) of three species of pine trees were compared at each of four locations, using samples of five trees per species at each location. The resulting data are given in the following...
-
Unlike affirmative action, diversity _ _ _ _ _ . a . can exist even if organizations do not take purposeful steps to create it b . is required by law for private employers with 5 0 or more employees...
-
What factors may impede the introductions of ABC?
-
Is it harder to transform the organization or the human resource? Explain.
-
is the differentiation strategy only appropriate for luxury goods? Explain.LO1
-
The financial statements of Marks and Spencer plc (M&S) are available at the books companion website or can be accessed at corporate.marksandspencer....
-
Assets are the same as expenses because they are acquired with cash always lower than liabilities financed by the stockholders and/or creditors equal to liabilities less stockholders' equity
-
A marketing firm is planning to conduct a survey of a segment of the potential product audience for one of its customers. The planning process for preparing to conduct the survey consists of six...
-
While working in the chemical stockroom, you discover an unlabeled bottle containing a liquid compound. You carefully smell the liquid and discover that it has a fishy odor. What functional group do...
-
The structure of a typical fat is shown here. Estimate the energy content of fat compared to the other compounds discussed in the Focus On box on p. 146 and explain yourreasoning. CH OC(CH) 16CH3 O...
-
Consider the following reaction to produce methyl acetate: When this reaction is carried out with CH3OH containing radioactive oxygen-18, the water produced is not radioactive. Explain. CH3OH +...
-
A. Use the following information to answer the six questions below. Variable Manufacturing Cost Per Unit20 Variable selling cost per unit25 Selling Price per unit100 Fixed Manufacturing cost per unit...
-
The team has been charged with reviewing quarterly results for the LusterLast moisturizing shampoo, called SatinSmooth. The product is new to the line and is sold mostly in drugstores and grocery...
-
Problem 4 (25 pts.) Consider the function f(x, y) = xy y +2. (i) (5 pts) Find the gradient of f (ii) (10 pts) Find the directional derivative of f at the point (1,2) in the direction of the vector...
-
PROBLEM 4. (15 points) a) Determine the range of charged particles emitted from Phosphorus-32 in iron. (5 points) b) Determine the necessary thickness of an iron plate to attenuate the flux of...
-
(b) In the case of no losses, Moody (1965) recommends the following equation for calculating the mass flow rate of wet steam (ie. two-phase water) through the constriction =A 2(h-h) Variable and...
-
What is the competitiveness of international destinations based on? LO.1
-
The column shown in the figure is fixed at the base and free at the upper end. A compressive load P acts at the top of the column with an eccentricity e from the axis of the column. Beginning with...
-
Sketch the curve r = cos 2.
-
Using an oxidative cleavage reaction, explain how you would distinguish between the following two isomericdienes: and
-
Compound A, C10H18O, undergoes reaction with dilute H2SO4 at 50 C to yield a mixture of two alkenes, C10H16.The major alkene product, B, gives only Cyclopentanone after ozone treatment followed by...
-
The cis and trans isomers of 2-butene give different Cyclopropane products in the Simmons?Smith reaction. Show the structure of each, and explain the difference. CH2I2, Zn(Cu) cis-CH3CH=CHCH3 CH2I2,...
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


