Question: Using Table 2.2, determine the number of covalent bonds that are possible for atoms of the following elements: germanium, phosphorus, selenium, and chlorine. Atomic Number
Using Table 2.2, determine the number of covalent bonds that are possible for atoms of the following elements: germanium, phosphorus, selenium, and chlorine.
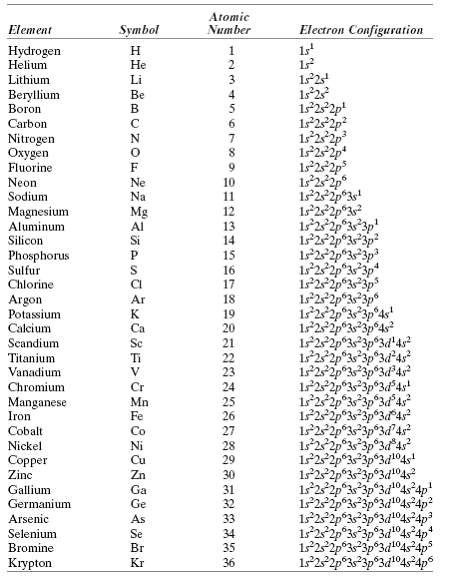
Atomic Number Electron Configuration Symbol Element 1s Hydrogen Helium He Lithium Li Beryllium Boron Be 4 1s22p! 1s22p 6. Carbon Nitrogen en Fluorine Neon Sodium 1s252p 1s2 2p 1s22p 1s22p3s! 1s22p3? Ne 10 Na 11 Magnesium Aluminum Silicon Mg 12 Al 13 122p 3p 14 Phosphorus Sulfur Chlorine 15 16 1s22 1s 22p 17 Argon Potassium 19 125 Calcium Scandium Titanium Vanadium Ca 20 21 22 23 24 25 26 27 1s2s 1252 Cr Chromium Mn Fe Manganese Iron 122 Cobalt Co Nickel 28 Copper Zinc 29 Zn 30 122p 1525 Gallium Ga 31 Germanium Ge 32 Arsenic As 33 Selenium Bromine Se 34 Br 35 122p33p3d14s4ps Krypton Kr 36
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
Germanium valence electron structure 4s 2 4p 2 N 4 therefore 8 N 4 covalent ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (21).docx
120 KBs Word File


