Question: Write and simplify the closed-system energy balance (Equation 7.3-4) for each of the following processes, and state whether nonzero heat and work terms are positive
Write and simplify the closed-system energy balance (Equation 7.3-4) for each of the following processes, and state whether nonzero heat and work terms are positive or negative. Begin by defining the system. The solution of part (a) is given as an illustration.
(a) The contents of a closed flask are heated from 25?C to 80?C.
(b) A tray filled with water at 20?C is put into a freezer. The water turns into ice at - 5?C. (Note: When a substance expands it does work on its surroundings and when it contracts the surroundings do work on it.)
(c) A chemical reaction takes place in a closed adiabatic (perfectly insulated) rigid container.
(d) Repeal part (c), only suppose that the reactor is isothermal rather than adiabatic and that when the reaction was carried out adiabatically the temperature in the reactor increased.
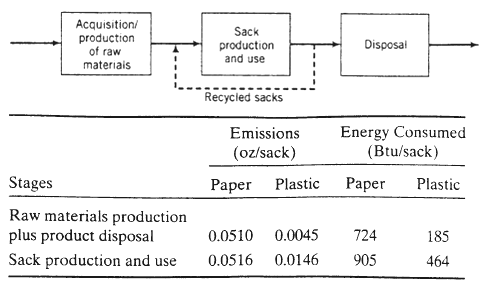
Acquisition/ production of raw materiais Stages Raw materials production plus product disposal Sack production and use Sack production and use Recycled sacks Emissions (oz/sack) Paper Plastic Disposal Energy Consumed (Btu/sack) Paper Plastic 0.0510 0.0045 724 0.0516 0.0146 905 185 464
Step by Step Solution
3.23 Rating (164 Votes )
There are 3 Steps involved in it
b QWAU AEkAEp AE 0 system is stationary AEp 0 no height ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (362).docx
120 KBs Word File


