Question: You have been assigned to collect thermodynamic data for a new liquid product your company is about to begin manufacturing and you decide to use
You have been assigned to collect thermodynamic data for a new liquid product your company is about to begin manufacturing and you decide to use a continuous-flow technique to generate a correlation of H versus T. You wrap an electrical heating tape around a pipe, cover the tape with a thick layer of insulation, pump the liquid through the pipe at the rate of 228 g/min. and adjust the power input to the heating tape with a variable resistor. For each resistance setting, you record the power input and the temperature of the liquid at the pipe outlet. You multiply the power input by a correction factor of 0.94 to determine the rate of heat input to the liquid. The entering fluid temperature remains at 25?C throughout the experiment. The following data are taken:
(a) Generate a table of 1(J/g) versus T(?C), taking 25?C and 1 atm as the reference state.
(b) Fit a line to the data (either graphically or by the method of least squares) to determine the coefficient b of an expression of the form ii = b(T ? 25).
(c) Estimate the heat input required to raise 350 kg/mm of the liquid from 20?C to 40?C.
(d) The correction factor of 0.94 accounts for the fact that the rate of energy input to the heating tape is somewhat greater than the rate of energy input to the liquid. Where does the additional energy go? (There are several answers.)
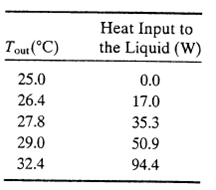
Tout (C) 25.0 26.4 27.8 29.0 32.4 Heat Input to the Liquid (W) 0.0 17.0 35.3 50.9 94.4
Step by Step Solution
3.46 Rating (175 Votes )
There are 3 Steps involved in it
a 228 gmin 25C QkW Energy balance OAH QW AEX 10 P 228 gmin TC Hout Jg 02630W TC 25 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (380).docx
120 KBs Word File


