Carbon dioxide dissolved in water makes the water slightly acidic. We desire to remove most of the
Question:
Carbon dioxide dissolved in water makes the water slightly acidic. We desire to remove most of the \(\mathrm{CO}_{2}\) from \(10.00 \mathrm{kmol} / \mathrm{h}\) of water containing \(\mathrm{x}_{0}=\) \(0.00001 \mathrm{~mol}\) fraction \(\mathrm{CO}_{2}\). The water enters at \(15^{\circ} \mathrm{C}\), the operation is isothermal, pressure is \(2.0 \mathrm{~atm}\), and the exiting water is at \(\mathrm{x}_{\mathrm{N}}=2.2 \times 10^{-7} \mathrm{~mol}\) fraction \(\mathrm{CO}_{2}\). The gas used for stripping out the \(\mathrm{CO}_{2}\) is pure air \(\left(\mathrm{y}_{\mathrm{N}+1, \mathrm{CO} 2}=\right.\) \(0)\). Henry's law constants are in Table 12-1.
Table 12-1
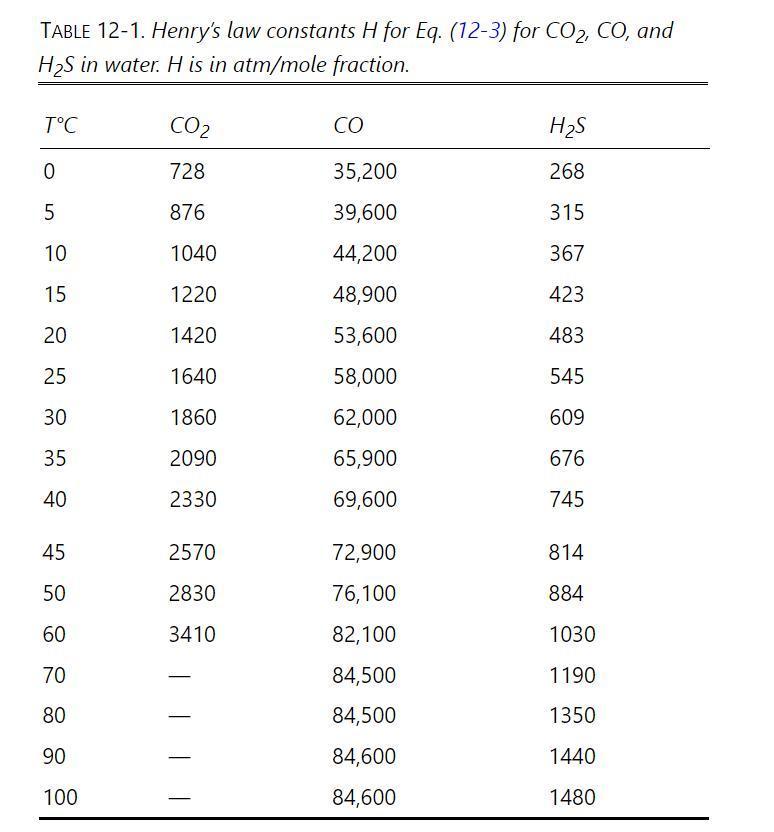
a. If gas flow rate \(\mathrm{V}=0.10 \mathrm{kmol} / \mathrm{h}\), what is \(\mathrm{y}_{1, \mathrm{CO} 2}\), the outlet gas mole fraction \(\mathrm{CO}_{2}\) ?
b. If \(\mathrm{V}=0.10 \mathrm{kmol} / \mathrm{h}\), how many equilibrium stages are required (include the fractional number)?
c. What is the minimum gas flow rate required?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





