(mathrm{HCl}) is being absorbed from two air streams into water in a countercurrent staged laboratory absorber at...
Question:
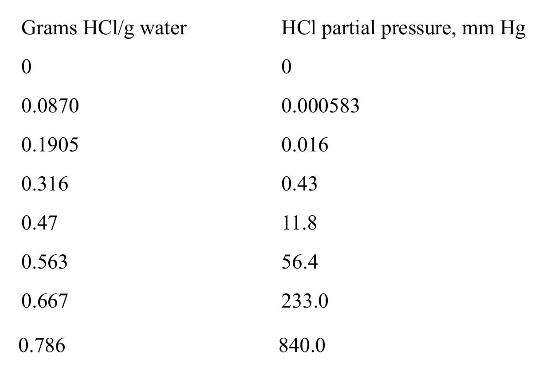
\(\mathrm{HCl}\) is being absorbed from two air streams into water in a countercurrent staged laboratory absorber at \(10.0^{\circ} \mathrm{C}\) and a pressure of \(2.0 \mathrm{~atm}\). Feed rate of gas feed 1 is \(1.0 \mathrm{kmol} / \mathrm{h}\) of total gas, and this gas is \(20.0 \mathrm{~mol} \% \mathrm{HCl}\). Feed rate of gas feed 2 is \(0.5 \mathrm{kmol} / \mathrm{h}\) of total gas, and this gas is \(5.0 \mathrm{~mol} \% \mathrm{HCl}\). Entering water is pure. Outlet gas should be \(0.2 \mathrm{~mol} \% \mathrm{HCl}\). Equilibrium data follow. Use optimum feed locations for both gas feeds.
a. Find the minimum water flow rate required.
b. If \(\mathrm{L}=0.4 \mathrm{~kg}\) water \(/ \mathrm{h}\), find the outlet liquid concentration, the number of stages required (including a fractional number of stages), and the optimum feed location for gas feeds 1 and 2.
Use ratio units. If careful with units, liquid units can be in mass and gas units in moles, which is effectively the form of the equilibrium data. Derive the operating equation and external mass balances to determine where to include the molecular weight of \(\mathrm{HCl}\) (36.46). Because \(\mathrm{HCl}\) has a very large heat of absorption in water, the column will have to be well cooled to maintain temperature at \(10.0^{\circ} \mathrm{C}\). Commercial units are not isothermal.
Equilibrium data from Perry and Green (1997, p. 2-127):
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





