We wish to absorb ammonia from an air stream using water at (0^{circ} mathrm{C}) and 1.3 atm.
Question:
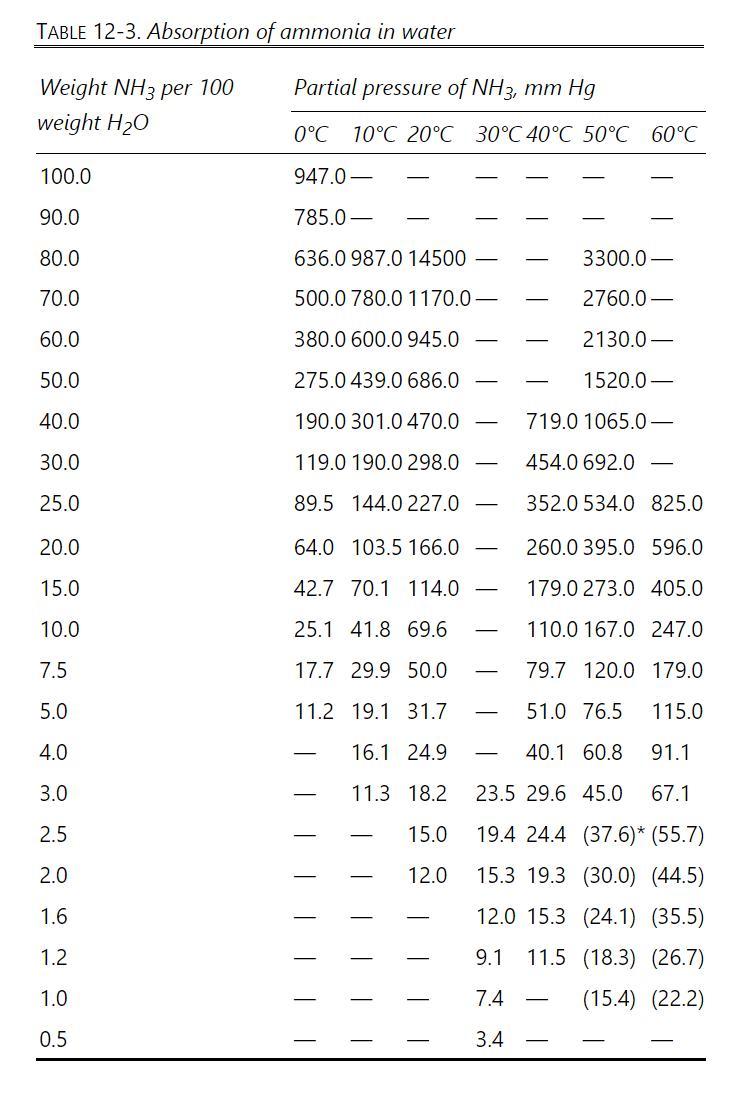
We wish to absorb ammonia from an air stream using water at \(0^{\circ} \mathrm{C}\) and 1.3 atm. Entering water stream is pure water, and entering vapor is \(17.2 \mathrm{wt} \%\) ammonia. Recover \(98.0 \%\) of the ammonia in the water outlet stream. Total gas flow rate is \(1050.0 \mathrm{~kg} / \mathrm{h}\). Use a solvent rate that is \(1.5 \times\) minimum solvent rate. Temperature is constant at \(0^{\circ} \mathrm{C}\), water is nonvolatile, and air does not dissolve in water. Equilibrium data are in Table 12-3. Find \(\mathrm{L}_{\min }\), L, and N.
Table 12-3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





